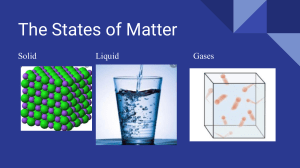
“SOLIDS, LIQUIDS & GASES” LESSON 1 - NOTE-TAKING SHEET Matter SOLID Matter is anything that takes up space and has mass. Mass is the amount of stuff in an object or substance Matter is made of tiny particles called atoms; Atoms are the basic building blocks of matter Matter is found in three different forms on Earth. We call these forms the states of matter. There three major states of matter are 1 solid 2 liquid 3 gas Each state of matter is made of particles that are packed together tightly, loosely or very loosely. Particle motion and particle forces determine a substance’s state of matter No matter how close they are to each other; all particles have random motion. Particles that are free to move will move in a straight line until they collide with something. There is a force of attraction between positively charged protons and negatively charged electrons. When particles move slower, they move closer together, and the attractive forces between them are strong. When particles move faster, they move farther apart, and the attractive forces between them are weaker. Description Shape/Volume Diagram Matter that is composed of atoms tightly packed together 1 The atoms are packed so tightly that they cannot move around but can vibrate 2 Has a definite shape & definite volume 1 A solid holds its shape & volume on its own 2 Solids The type of solid depends on how the particles in the solid are arranged. If the particles are randomly arranged, the solid is an amorphous solid. When the particles are arranged in a specific, repeating order, the solid is a crystalline solid. Description Shape/Volume Matter that is composed of atoms loosely packed together. 2 There is some space between the atoms so the atoms can slightly move around 3 As particles move farther apart, attractive forces decrease 1 Liquids have definite volume but DO NOT have definite shape 1 LIQUI D Diagram Liquids take the shape of their container 2 Liquids A measurement of a liquid’s resistance to flow is its viscosity The attraction between molecules that are alike, such as water molecules, is called cohesion. Molecules at the surface of a liquid also have surface tension, which involves the uneven forces acting on the particles on the surface of a liquid. Description Shape/Volume Matter that is composed of atoms very loosely packed together 1 GAS 1 There is a lot of space between the atoms & they are constantly moving around 2 Diagram Gases have NO definite volume & NO definite shape Gases take the shape & volume of their container 2 Gases The gas state of a substances that is usually a solid or a liquid at room temperature is called a vapor Plasma - 4th state of matter. Most common state of matter in the Universe, not commonly found on Earth; can be manmade Plasma is high-energy matter consisting of positively & negatively charged particles; most like a gas 1 2 3 EXAMPLEs: neon signs plasma ball lightning 4 5 6 northern lights (AKA aurora) sun plasma TV “SOLIDS, LIQUIDS & GASES” LESSON 1 - NOTE-TAKING SHEET Matter SOLID Matter is anything that __________________________ and has ___________. Mass is the ______________________________in an object or substance Matter is made of ________________________called ____________; Atoms are the basic ________________________________ Matter is found in ________ different forms on Earth. We call these forms the ________________________________ There three major states of matter are 1 ___________ 2 ___________ 3 ________ Each state of matter is made of particles that are packed together ________________, loosely or ________________________. Particle ________ & particle ________ determine a substance’s ____________________ No matter how close they are to each other; all particles have ___________________ Particles that are free to move will move in a ______________________ until they _________________with something. There is a _____________of ___________________between __________________charged _____________and _____________________charged __________________. When particles move _____________, they move ________________together, and the _____________________forces between them are ________________. When particles move _____________, they move ______________________, and the attractive forces between them are __________________. Description Shape/Volume Diagram Matter that is composed of _______________________ __________________________ 2 The atoms are packed so tightly that _____________ __________________________ __________________________ 1 Has a ________________ & _____________________ 1 A solid ______________ _____& ________________ _______________________ 2 Solids The type of solid depends on how the ______________in the solid are ______________ If the particles are _______________arranged, the solid is an ________________________solid When the particles are arranged in a _______________, __________________________, the solid is a _____________________solid. LIQUID Description Shape/Volume 1 Matter that is composed of atoms _____________________ 1 ____________________ 2 There is ________________ between the __________ so the atoms can _________________ ____________________ ______________________ Diagram Liquids have __________ __________but _________ 2 Liquids _______________ _______________________ ______________________ As particles move farther apart, ______________ forces decrease 3 Liquids A measurement of a liquid’s _________________________is its ____________________ The attraction between molecules that are alike, such as ____________molecules, is called ___________________ Molecules at the ______________ of a liquid also have ________________________, which involves the ____________ ______________acting on the particles on the _______________ Description Shape/Volume Matter that is composed of atoms ________________ 1 GAS 1 _________________________ 2 There is a ______________ __________________________ & they are ______________ __________________________ Diagram Gases have ___________ ___________ & ___________ ______________________ Gases ________________ ________& ______________ _________________ 2 Gases The gas state of a substances that is usually a ___________ or a ___________ at ______________________ is called a ___________ Plasma - ______state of matter. Most common state of matter in the __________________, not commonly found on _______________; can be _______________ Plasma is _______________________ matter consisting of ____________________& _____________________charged particles; Plasma is most like a ___________ 1 EXAMPLEs: _______________ 2 ____________________ 3 ________________ 4 ______________________ (AKA_____________) 5 _______ 6 _________________