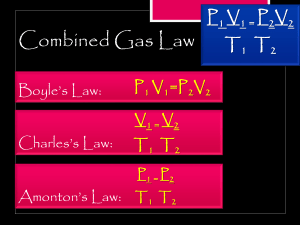
Boyle’s Law Problems P1V1=P2V2 1. A 2.5 L container has a gas pressure of 4.6 atm. If the volume is decreased to 1.6 L, what would be the new gas pressure inside the container 2. The gas inside a flexible 3.5L container has a pressure of 115 Kpa. What should the volume of the container be increased to in order to decrease the pressure to 625 torr? 101.325 KPa = 760 torr 3. The volume of a gas at 17.5 psi decreased from 1.5 L to .75L (750mL). What is the new pressure of the gas in atm? 14.7 psi = 1 atm