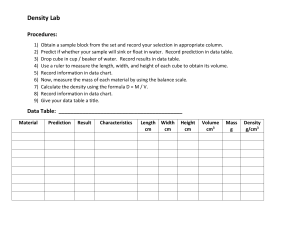
Week 1 Chapter 1CHEM – Introduction to Essential Ideas Chemistry – The study of the composition, properties, and interactions of matter. The Scientific Method o Hypothesis: Tentative explanation of observations that acts as a guide for gathering and checking information. o Theory: Well-substantiated, comprehensive, testable explanation of a particular aspect of nature. o Law: Statement that summarizes a vast number of experimental observations, and describes or predicts some aspect of the natural world. The Domains of Chemistry o Macroscopic Domain Everything that can be directly observed in the everyday world, such as density, solubility, and flammability. o Microscopic Domain Everything that needs additional tools to be seen, such as microscopes to view bacteria. Atoms and molecules are too small to be seen, but they are still very real. o Symbolic Domain Uses specialized language to represent components of the macroscopic and microscopic domains. These include chemical symbols and chemical equations. - An example of these domains is water. Water can be directly observed in the macroscopic domain. Properties can be described as H2O, which enters the symbolic domain. Descriptions of water freezing and boiling, and the way its molecules change, enters the microscopic realm. Phases and Classification of Matter Matter: Anything that occupies space and has mass. o Solids: Rigid and possesses a definitive shape. o Liquids: Flow and take the shape of a container; takes a flat appearance when acted upon by gravity o Gases: Takes both the shape and volume of its container. o Plasma: A gas that contains an appreciable number of electrically charged particles. Mass: The measure of the amount of matter in it. Weight: The force that gravity exerts on an object The Law of Conservation of Matter There is no detectable change in the total quantity of matter present when matter converts from one type to another type (via chemical change) or changes among solid, liquid, or gaseous states (physical change). o For example, when beer is brewed, there is no loss of substance even though the yeast has taken sugar and digested it into ethanol. Using the Law of the Conservation of Matter o Example: A sample of mercury with a mass of 114.0 g was combined with 12.8 g of oxygen gas and the resulting reaction gave 123.1 g of mercury oxide. How much oxygen was left over after the reaction is complete? Atoms and Molecules Atoms o The smallest particle of an element that has the properties of that element and can enter into a chemical combination o Dalton’s Atomic Theory Molecules o Two or more atoms joined by strong forces called chemical bonds. Classifying Matter Pure Substances o Homogeneous substance that has a constant composition, such as sucrose. Any sample of sucrose consists of 42.1% carbon, 6.5% hydrogen, and 51.4% oxygen by mass, and will always have the same physical properties. o Pure substances that cannot be broken down into simpler substances are called elements, such as iron and gold. o Pure substances that can be broken down are called compounds, such as sucrose or mercury oxide. Mixtures: composed of two or more types of matter than can be present in varying amounts and can be separated by physical changes, such as evaporation. o Heterogeneous Mixture: combination of substances with a composition that varies from point to point. Example is Italian dressing, where the olive oil and vinegar remain separate. o Homogeneous Mixture: (also, solution) combination of substances with a composition that is uniform throughout. Examples are sodas, syrup, and gasoline. Decomposition of Water/ Production of Hydrogen Water consists of the elements hydrogen and oxygen in a 2-1 ration. Water can be broken down intro hydrogen and oxygen gases by the addition of energy. Physical and Chemical Properties The characteristics that enable us to distinguish one substance from another are called properties. o A physical property is a characteristic of matter that is not associated with a change in its chemical composition. Ex. Density, color, hardness, melting and boiling points. A physical change is a change in the state or properties of matter with no change to its chemical composition (i.e. ice melting into water) o A chemical property is the change of one type of matter into another type (or the inability to change). Examples are flammability, acidity, reactivity. (i.e., iron combines with oxygen in the presence of water to form rust) Two categories of properties o Extensive properties: The property changes depending on the amount of the substance (mass, volume) o Intensive properties: No matter the amount of the substance, the property stays the same (temperature) Hazard Diamond Periodic Table Measurements Chemistry uses the metric system for its calculations. These units can be presented as fractions or multiples of the base unit. Derived SI Units o Can use base units to define different types of units, such as length to define volume in a space, or combine mass and length to define a unit of density. In chemistry, you will need to understand how to convert units. Convert the volume 3.5 ft3 to cm3. Remember to always square/cube your conversion factor! The 12 in^3 becomes 6048 in^3, and the 2.54 cm^3 becomes 16.39 cm^3. The density of a substance is the ratio of the mass of a sample of the substance to its volume. The SI unit for density is the kilogram per cubic meter (kg/m3). For many situations, however, this as an inconvenient unit, and we often use grams per cubic centimeter (g/cm3) for the densities of solids and liquids, and grams per liter (g/L) for gases. Although there are exceptions, most liquids and solids have densities that range from about 0.7 g/cm3 (the density of gasoline) to 19 g/cm3 (the density of gold). The density of air is about 1.2 g/L. Table 3 shows the densities of some common substances. Density is found with the formula that divides mass by volume. Measurement Uncertainty, Accuracy, and Precision Significant Figures in Measurement o Significant figures: All the measured digits in a determination, including the uncertain last digit. o Uncertainty: Estimate of amount by which measurement differs from true value o Placeholder (come back to this later, read Dummies book) Accuracy and Precision o Accuracy: How closely a measurement aligns with a correct value o Precision: How closely a measurement matches the same measurement when repeated.