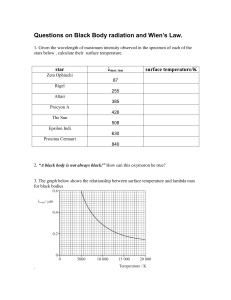
PHY310/March 2020 – July 2020 UNIVERSITI TEKNOLOGI MARA CAWANGAN PERLIS PHY 310 MODERN PHYSICS LABORATORY REPORT Experiment No. :1 Title :BLACKBODY RADIATION Program / Group : AS1204G Date of Experiment : 20/04/2020 Lecturer : Dr. Mohd Nazari Abu Bakar Name Matrix No Nur Arinah Binti Mohamad 2018445848 Objective 1. To establish relationship between temperature, Wien’s Law and Stephan-Boltzmann. 2. To create understanding of how thermal spectra can be used to evaluate the temperature of an object. Theory Blackbody radiation is an important phenomenon when studying thermal radiation and electromagnetic radiation energy transfer in all wavelength bands. Blackbody itself is an ideal object that absorb all incident light hence the name ‘black’. This ideal radiation absorber is used as a standard to compare radiation emitted by real physical bodies. Using known formalities such as Stefan’s Law and Wien’s Displacement Law, the energy transferred to environment and the peak wavelength can be calculated. The peak emission of this radiation depends on the temperature. The hotter an object is the more radiation it emits at shorter wavelengths with more energy i.e. at higher frequency. Method 1. The link https://phet.colorado.edu/sims/html/blackbody-spectrum/latest/blackbody spectrum_en.html is clicked to open the simulation. 2. Once the simulation is open, try to play with it. 3. The temperature is set to the temperature of the Sun. At this temperature, the peak wavelength is found and the colour (in the visible range) is determined. Compare this colour and the colour of the sunlight. Is there is any difference? Why? 4. The camera button is clicked to save the Sun spectrum. Next, the temperature slider is changed to 7000 K and 8000 K. The temperature, peak wavelength, and power intensity at each temperature is recorded. These values is put in the table by using the Wien’s Law and Stefan-Boltzmann’s Law equations. Then, the error percentage is calculated. Result/data Peak wavelenght, peak (nm) Temperature, T (K) Intensity, I (W/m4) Simulation Calculated Simulation Calculated 5800 500 500 6.42 107 6.42 107 7000 414 414 1.36 108 1.36 108 8000 362 362 2.32 108 2.32 108 Calculation Peak wavelenght, λpeak Using Wien’s Law λmaxT = 2.898 x 10-3m.K 1. T= 5800K λmax(5800K) = 2.898 x 10-3m.K λmax= 2.898 x 10-3m.K / 5800K = 5.0 x 10-7m / 10-9 λmax= 500 nm 2. T= 7000K λmax(7000K) = 2.898 x 10-3m.K λmax= 2.898 x 10-3m.K / 7000K = 4.14 x 10-7m / 10-9 λmax= 414 nm 3. T= 8000K λmax(8000K) = 2.898 x 10-3m.K λmax= 2.898 x 10-3m.K / 8000K = 3.62 x 10-7m / 10-9 λmax= 362 nm Intensity, I (W/m4) Using Stefan-Boltzman Law S= σT4 1. T= 5800K S= 5.6703 x 10-8 W/m2 k4 (5800K)4 = 6.42 x 107 W/m2 2. T= 7000K S= 5.6703 x 10-8 W/m2 k4 (7000K)4 = 1.36 x 108 W/m2 3. T= 8000K S= 5.6703 x 10-8 W/m2 k4 (8000K)4 = 2.32 x 107 W/m2 Analysis Data Percentage Error %error | simulated calculated | 100 calculated From graph λ simulation vs λ calculated, yx where, simluated calculated | simulated calculated | 100 calculated %error 0% %error From graph I simulation vs I calculated, yx where, Isimluated Icalculated | simulated calculated | 100 calculated %error 0% %error Discussion 1. Compare the colour that you found in method no.3 and the colour of the sunlight. Is there is any difference? Why? The sun's spectrum peaks in the green wavelengths.Therefore, the colour shows it is green while the color of sunlight that we see is yellow-red. There is a huge different. This is because,the truth to be told that when we're above the sensible atmosphere we see sunlight as white because the sun emits all colors of the rainbow more or less evenly and in physics, we call this combination "white". But our eyes don not isolate the peak color so we could not see the green color appears by our naked eyes. If sunlight were purely green, then everything outside would look green or dark. The sun emits all colors of visible light, and in fact produces all frequencies of electromagnetic waves except gamma rays. This includes radio waves, microwaves, infrared waves, visible light, ultraviolet waves, and X-rays. The sun emits all these colors because it is a thermal body and emits light through the process of thermal radiation. The atmosphere scatters short wavelengths much more effectively than long wavelengths. This is why we get those lovely red sunsets where all the air between our eyes and the setting Sun scatters away so much of the blue end of the "rainbow" that what's left is largely red. 2. If the body of the human body is around 37°C, can we see the light coming from the body? Why? Use the simulation to support your answer. We cannot see the light coming from our body as if the human body temperature is 310.15K. This can explained through the simulation that have been conducted. Based on the diagram of the simulation, its peak wavelenght lies in the infrared range. Human vision can see red light wavelengths as long as about 700 nm. The infrared wavelengths are generally defined as being between 700 and 100,000 nm. Infrared light, is electromagnetic radiation with wavelengths longer than those of visible light. It is therefore generally invisible to the human eye, although infrared at wavelengths up to 1050 nm from specially pulsed lasers can be seen by humans under certain conditions only. Conclusion In conclusion, from this experiment we can see that when the temperature increases, its peak wavelenght and intensity decreases. I have better understanding on blackbody radiation while doing this simulation as I can understand clearer that different temperature has its different peak wavelenght where the spectrum reaches its highest intensity.