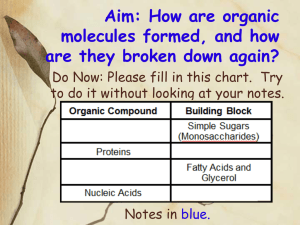
SBI4U Hydrolysis and Dehydration Synthesis: Biologically Important Chemical Reactions Important terms: Monomer- single molecule or “building block” that can form polymers Polymer- long/large molecule of repeating monomers Polymerization- reactions forming a polymer All chemical reactions within a living organism can be classified as: Anabolic reaction- chemical reactions in which simpler substances are combined to form more complex molecules. Anabolic reactions usually require energy. Anabolic reactions build new molecules and/or store energy. Catabolic reaction- chemical reactions that result in the breakdown of more complex organic molecules into simpler substances. Release energy that is stored (in ATP) or used to drive anabolic reactions. Hydrolysis A chemical reaction that results in cleavage of a covalent bond with the addition of a water molecule (Hydro = water; lysis = break); a reaction process that breaks covalent bonds between monomers by the addition of water molecules. A hydrogen from the water bonds to one monomer and the hydroxyl bonds to the adjacent monomer. The covalent bond between these monomers breaks and the larger molecule (polymer) is split into smaller molecules (monomers) Releases energy Dehydration Synthesis *Note: your textbook describes Dehydration Synthesis as “Condensation” A chemical reaction that results in the formation of a covalent bond between two molecules (“synthesis”) by removing a water molecule (“dehydration”) One monomer loses a hydroxyl (–OH), and the other monomer loses a hydrogen (–H). (the H usually comes from a functional group) Process requires energy. - Process requires biological catalysts or enzymes. Each of the four macromolecules (Carbohydrates, Lipids, Proteins and Nucleic Acids) use dehydration synthesis reactions to build polymers and hydrolysis reactions to break polymers into monomers.