Intermolecular forces quiz combined
advertisement
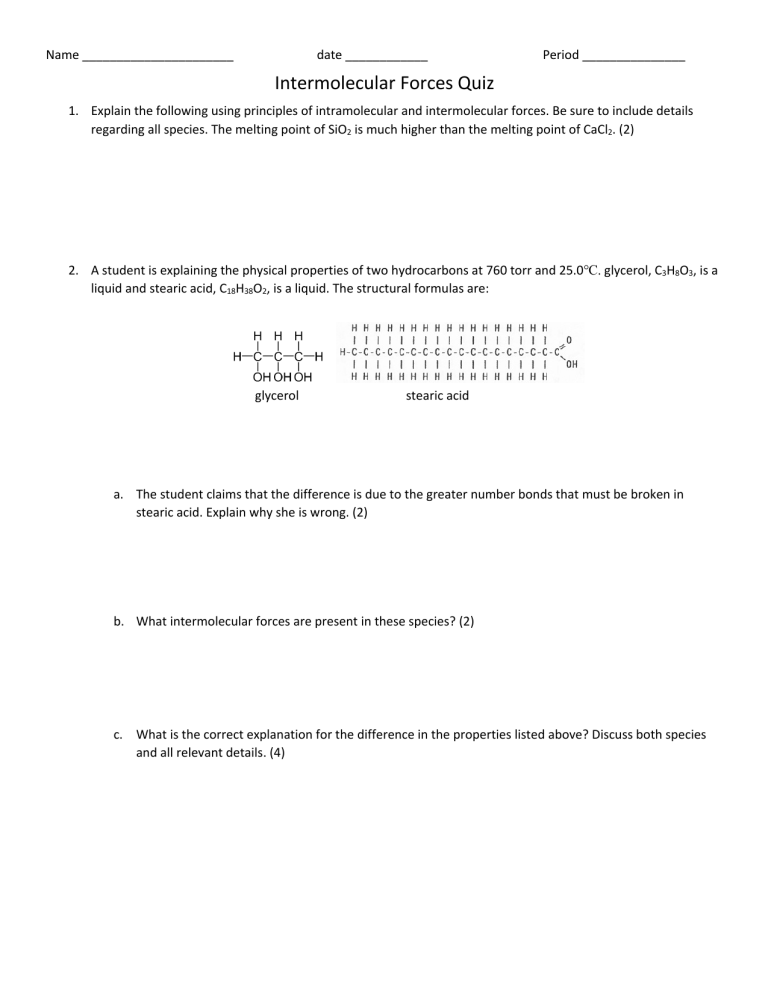
Name ______________________ date ____________ Period _______________ Intermolecular Forces Quiz 1. Explain the following using principles of intramolecular and intermolecular forces. Be sure to include details regarding all species. The melting point of SiO2 is much higher than the melting point of CaCl2. (2) 2. A student is explaining the physical properties of two hydrocarbons at 760 torr and 25.0℃. glycerol, C3H8O3, is a liquid and stearic acid, C18H38O2, is a liquid. The structural formulas are: glycerol stearic acid a. The student claims that the difference is due to the greater number bonds that must be broken in stearic acid. Explain why she is wrong. (2) b. What intermolecular forces are present in these species? (2) c. What is the correct explanation for the difference in the properties listed above? Discuss both species and all relevant details. (4) Name ______________________ date ____________ Period _______________ Intermolecular Forces Quiz 1. Explain the following using principles of intramolecular and intermolecular forces. Be sure to include details regarding all species. The melting point of SiC is much higher than the melting point of NaCl. (2) 2. A student is explaining the physical properties of two hydrocarbons at 760 torr and 25.0℃. Propane, C3H8, is a gas and octane, C8H18, is a liquid. The structural formulas are: a. The student claims that the difference is due to the greater number bonds that must be broken in octane. Explain why she is wrong. (2) b. What intermolecular forces are present in these species? (2) c. What is the correct explanation for the difference in the properties listed above? Discuss both species and all relevant details. (4)





