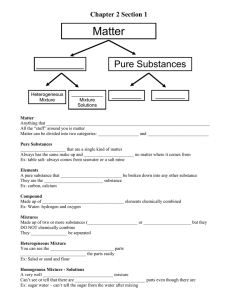
Name: _________________________________________ Date: _____________________ Period: __________ Pure substances vs. Mixtures For the following: On the first blank write if the example is a pure substance or mixture. If it is a pure substance, then on the second blank write if the example is an element or compound. If it is a mixture, then on the second blank, write if the example is a heterogeneous mixture or homogeneous mixture. 1. _____ ____________Hydrogen 14. _____ ____________Milk 2. _____ ____________Hydrogen gas 15. _____ ____________Chocolate milk 3. _____ ____________Copper 16. _____ ____________Chocolate chip cookie 4. _____ ____________Brass 17. _____ ____________Water 5. _____ ____________Iron 18. _____ ____________Salt water 6. _____ ____________Steel 19. _____ ____________Sugar water 7. _____ ____________24 karat gold 20. _____ ____________Soda 8. _____ ____________18 karat gold 21. _____ ____________Italian salad dressing 9. _____ ____________14 karat gold 22. _____ ____________Caesar salad 10. _____ ____________Kool aid 23. _____ ____________Air 11. _____ ____________ Raisin bran 24. _____ ____________Oxygen 12. _____ ____________ Trail mix 25. _____ ____________Oxygen gas 13. _____ ____________Glucose (C6H12O6) 26. _____ T/F Pure substances are either heterogeneous or homogeneous mixtures. 27. _____ T/F Substances composed of two or more elements combined chemically in a fixed proportion by mass are called compounds. Complete the diagram below with the following terms: matter, pure substance, mixture, element, compound, homogeneous mixture & alloys, heterogeneous mixture. 1. 2. 4. 3. 5. 6. 7.