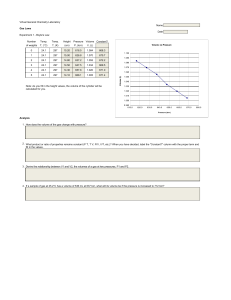
Gay-Lussac’s Law Problems *Hint make sure the units are always in Kelvin before doing the question* 1. Find the missing values within the table: a. b. c. P1= 14atm T1= 273.15K P2= 19atm T2= ? K P1= 890mmHg T1= ? K P2= 760mmHg T2= 101K P1= T1= 340K P2= ? kPa T2= 100K T1= 380K P2= 800Torr T2= 120K 101.325kPa d. P1= ?Torr 2. Nitrogen in a container kept at 690 Torr is heated from 400K to 600K, what must the new pressure of the gas be? 3. A sample of nitrogen at a temperature of 353 K with a pressure of 150kPa is reduced to a pressure of 120kPa, because of a temperature change. What is the final temperature of the gas in kelvin? 4. A sample of argon kept at 860mmHg was heated at 300K to 760K, what is the final pressure of the argon? 5. A student within a lab at 101.325kPa decided to heat a neon sign at standard ambient temperature (298.15K) to 500K exploding the neon sign, what would have been the theoretical pressure of the final gas? 6. A scientist collects a solution of helium at 940 Torr in a balloon at 250K, if the temperature drops to 90K what is the new pressure of the gas? Gay-Lussac’s Law Problems 7. 5atm of pressure is applied to a gas at 230K, if the pressure is increased to 10 atm what is the final temperature? 8. a. b. c. d. P1= 224 kPa T1= 13OC P2= 2.8atm T2= ? K P1= 780mmHg T1= ? K P2= 16atm T2= 40OC P1= ?Pa T1= 40OC P2= 4atm T2= 280K P1= 115000Pa T1= 50OF P2= ? Pa T2= 400K 9. When a gas at 29 OC is compressed from 860Torr to 760Torr, what is the final temperature in K. 10.When a balloon of helium at 25OC on the ground at 1 atm is let go it reaches the atmosphere where the temperature decreases to 275K, what is the new pressure of the gas in the balloon in Pa? 11. If a gas is at 870K but the pressure increased from 2atm to 2300Torr=what is the final temperature in OF? Answer Key: 1. a. 371 K, b. 118 K, c. 29.8 Pa, d. 2533 Torr 2. 1035 Torr 3. 282.4 K 4. 2179 mmHg 5. 169.923 kPa 6. 338 K 7. 460K 8. a. 362K, b. 20K, c. 453285Pa, d. 162458Pa 9. 267K 10. 93458Pa 11. 1910OF