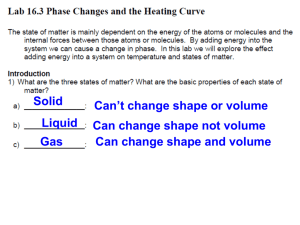
Name:_________________ Water Phase Change Lab Phase changes are happening all around you. The water vapor traveling through radiators to warm your house, the water in hot coffee evaporating, and even the sweat evaporating off one’s skin when the wind blows are all examples of phase changes. All of these phase change examples require a transfer of energy. Energy has several different forms including mechanical, radiant, sound, chemical, and heat (electric and nuclear are also forms of energy). Heat energy is the amount of kinetic energy any given particle or atom has at that instant. Energy, or heat, needs to be transferred into a system or out of a system for phase changes to occur. Look at the picture below. Today you will conduct an experiment that asks the following question: What is the relationship between the time it takes for liquid water to become completely frozen and temperature ? Hypothesis: I think (insert claim)… because…(insert reasoning) _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ HONORS CHEMISTRY READ THIS SECTION! A typed and complete lab report (50 points) is expected to be done by the student independently, with a partner, or with two partners. Three persons per group is the maximum amount of people per group. The procedure is located on the back of this sheet. An example of a well written lab report will be provided by the instructor. A rubric will also be provided to help organize and inform students what is expected in their written lab report. You will not be handing in this packet. Follow the procedure that is located on the back of this sheet to conduct a successful experiment. If you have any questions during the experiment, please ask your instructor for clarification. Good luck and remember, YOU are the scientist. REGULAR CHEMISTRY READ THIS SECTION! It is your goal to complete the lab and hand in this packet with a completed hypothesis, data table, observations, graph, calculations, and an evidence based conclusion where you reason your data. Good luck and remember, YOU are the scientist. Procedure 1. Obtain one 200mL or 300mL and fill it 1/2 full of ice that is located in the freezer. 2. Measure out 50.0mL of water and pour into the beaker with the ice. 3. Pour 1 full spoon full (Tablespoon) of rock salt into the ice water. Salt water has a lower freezing temperature than pure water due to the energy needed by the water to separate the salt’s ions. Gently shake the ice, water, and salt to mix the contents. 4. Obtain a test tube and a small graduated cylinder. Measure out 10.00mL of water with the graduated cylinder and pour the water into the test tube. Attach the test tube securely to the ring stand with the test tube holder. 5. Obtain a thermometer and a timer. The student may use one’s phone for a timer. 6. Place the thermometer into the test tube and record the initial temperature. 7. Then place the test tube, with the thermometer included, into the beaker full of icy salt water. Make sure the thermometer is visible to record temperature measurements and do not touch adjust or touch the thermometer. 8. Start the timer starting at 0:00 seconds. 9. The temperature of the water should begin to decrease. Record the exact temperature (estimate the last measurement digit) every THIRTY SECONDS for TWENY MINUTES in the data table below. Time (minutes) 0.0 (Ti) Temperature (Celsius) Time (min) 5.0 Temperature (C) Time (min) 10.0 Temperature (C) Time (min) 15.0 0.5 5.5 10.5 15.5 1.0 1.5 6.0 6.5 11.0 11.5 16.0 16.5 2.0 7.0 12.0 17.0 2.5 7.5 12.5 17.5 3.0 8.0 13.0 18.0 3.5 8.5 13.5 18.5 4.0 9.0 14.0 19.0 4.5 9.5 14.5 19.5 20.0 Temperature (C) Observations: Record observations during the lab below. __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ Graph: Below, plot the data (line graph) for time energy was released (x-axis) and the temperature (y-axis). Also, please HIGHLIGHT the section of your temperature curve where freezing took place. _____________________ Calculations: Please calculate the amount of energy LOST by the 10.0mL of water from 0 seconds to 25 minutes. This involves a combination of the specific heat formula (q=mc∆T) and latent heat formula (q=mHfus). Make sure you include the correct units for all measurements, constants, and calculated answers. Conclusion: Below, use evidence from your investigation to answer the original question: “What is the relationship between the time it takes for liquid water to become completely frozen and temperature? ” Remember to reference your graph and observations when writing your conclusion. This is how a scientist uses evidence to provide a reason for their claim. __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________