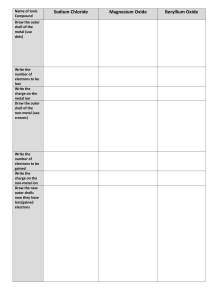
b The table has seven rows and 18 columns. Each row represents one period; the period number of an element indicates how many of its energy levels house electrons. Sodium, for instance, sits in the third period, which means a sodium atom typically has electrons in the first three energy levels. Moving down the table, periods are longer because it takes more electrons to fill the larger and more complex outer levels. The columns of the table represent groups, or families, of elements. The elements in a group often look and behave similarly, because they have the same number of electrons in their outermost shell — the face they show to the world. Group 18 elements, on the far-right side of the table, for example, have completely full outer shells and rarely participate in chemical reactions. DOWN A GROUP- Electrons are further from nucleus (more shielding) less controlled by nucleus ACROSS A PERIOD-More electron are being added to the same shell: so tighter control Metal reactivity increases down a group (easier to lose e-) Non-metals are also more likely to react in a way as to hold on to e- Group A- elements are sometimes called the representative elements because they exhibit the full range of chemical properties Group B - these are the transition elements - elements that are filling their d orbitals and display a range of stable and reactive cation states