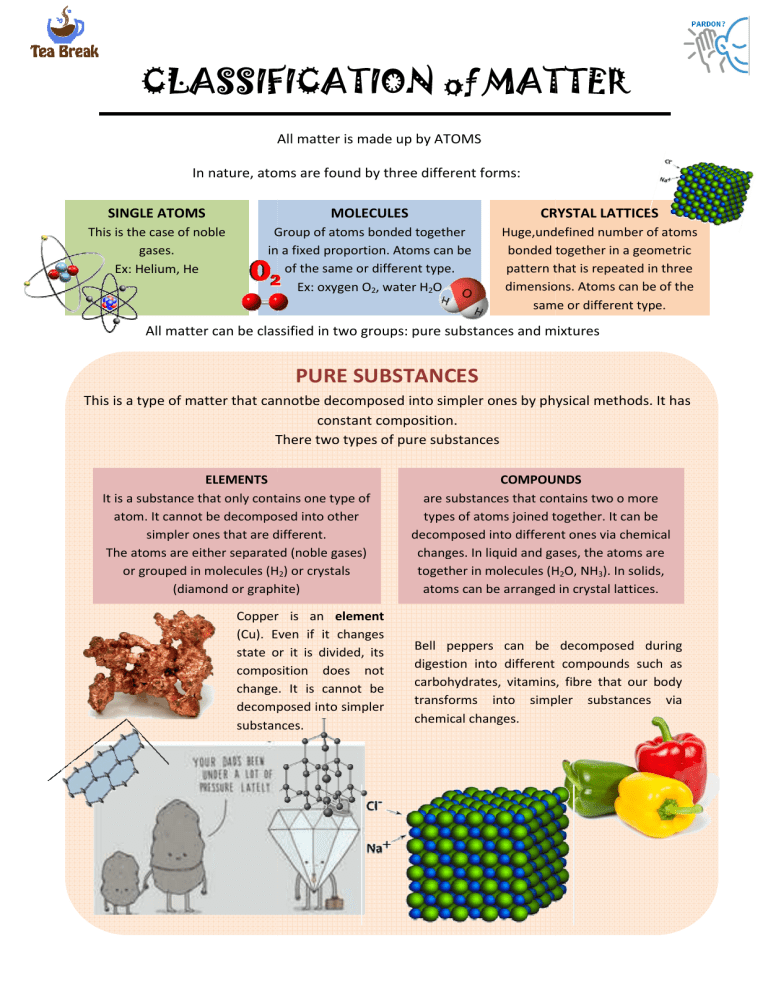
CLASSIFICATION of MATTER All matter is made up by ATOMS In nature, atoms are found by three different forms: SINGLE ATOMS MOLECULES CRYSTAL LATTICES This is the case of noble gases. Ex: Helium, He Group of atoms bonded together in a fixed proportion. Atoms can be of the same or different type. Ex: oxygen O2, water H2O Huge,undefined number of atoms bonded together in a geometric pattern that is repeated in three dimensions Atoms can be of the dimensions. same or different type. All matter can be classified in two groups: pure substances and mixtures PURE SUBSTANCES This is a type of matter that cannotbe decomposed into simpler ones by physical methods. methods It has constant composition. There two types of pure substances ELEMENTS It is a substance that only nly contains one type of atom. It cannot be decomposed into other simpler ones that are different. The atoms are either separated (noble gases) or grouped in molecules (H2) or crystals (diamond or graph phite) Copper is an element (Cu). Even if it changes state or it is divided, its composition does not change. It is cannot be decomposed into simpler substances. COMPOUNDS are substances that contains two o more types of atoms joined together. It can be decomposed into different ones via chemical changes. In liquid and gases, the atoms are together in molecules (H2O, NH3). In solids, atoms can be arranged in crystal lattices. Bell peppers can be decomposed during digestion into different compounds such as carbohydrates, vitamins, fibre that our body transforms into simpler substances via chemical changes. QUESTIONS 1. Are the following particles elements or compounds? a. A water molecule b. An oxygen molecule c. A neon atom 2. Are these crystal elements or compounds? a. Salt b. Diamond c. Copper sulphate crystals (CuSO4) 3. Are these statements true or false? a. An element can be formed of molecules or crystals b. A compound can be made of single atoms 4. Look at the pictures and decide whether they correspond to an element or a compound.Use expressions like: - I think is an element because... - This must be a compound because... - I can see here two different atoms bond together so... 5. Look at the picture and say what each one is a. A compound that forms a crystal lattice b. A monoatomic element c. An element that forms a crystal lattice d. A compound that forms a molecule.




