1
CRIMEAN STATE MEDICAL UNIVERSITY
NAMED AFTER S.I.GEORGIEVSKY
Digest on pathomorphology
Professor
ALEXANDR ZAGOROULKO
Assistant of professor
TATYANA FILONENKO
Crimea, Simferopol
2007
2
УДК 616-091
Z 16
Рецензенти
І.В.Задніпряний – д.м.н., професор кафедри анатомии КДМУ ім. С.І.Георгієвского
О.Ю.Шаповалова – д.м.н., профессор, завідувач кафедри гістології КДМУ ім. С.І.
Георгієвского
Друкується в авторскій редакції.
О.Загорулько, Т.Філоненко
«Дайджест з патоморфології». – Сімферополь, 2007.-417с. – Мова англ.
ISBN 966-73348-14-8
«Дайджест з патоморфології» (друге видання) підготовлений Академіком Міжнародної
Академії Патології, завідувачем кафедри патоморфології Кримського державного медичного
університету Олександром Загорулько і доцентом кафедри Тетяною Філоненко. Книга містить
короткий огляд головних тем з загальної і клінічної патоморфології відповідно до програми,
затвердженої Центральним методичним кабінетом вищої освіти Міністерства охорони здоров’я
України. Книга розрахована на студентів медичних вузів, які навчаються англійською мовою.
Z 143
Z 143
A.Zagorоulko, T.Filonenko
«Digest on pathomorphology». – Simferopol, 2007. – 417 p.
ISBN 966-73348-14-8
“Digest on pathomorphology” (second edition) is prepared by Academician of International
Academy of Pathology, Head of the Department of Pathology of the Ctimean State Medical
University, professor Alexander Zagoroulko, PhD, MD and assistant of professor Tatyana Filonenko,
PhD. The book includes the quick review of the main topics on general and systemic
pathomorphology.
All rights reserved. This book is protected by copyright. No part of this book may be
reproduced in any form or by any means, including photocopying, or utilized by any information
storage and retrieval system without written permission from the copyright owner.
3
Preface
In the preface to the first edition we stated our motive as follows: “We believe that
communication by verbal and written methods are fundamental basis for study and lerning.
Nevertheless, in the mordern setting where knowledge increases so rapidly and in subject such as
pathology where morphological changes are a major component, we consider that the quick review
has an important facilitating role”.
The first edition of the present book is abridged information about the main topics of the
pathomorphology, which combined the efforts of the scientific achievements of the all
pathomorphologists as in theUkraine and other countries as well.
We have attempted to extract the essential elements from the various pathomorphological
literatures for facilitation of the understanding of pathomorphology. Because pathology is the basis of
our medical practice, or, in the words of Sir William Osler, “As is our pathology, so is our practice.”
This book is expected to fulfil the following goal: as an aid to students to revise the subject
quickly near the examinations in short period of time.
4
PART I. GENERAL PATHOLOGY
INTRODUCTION ON PATHOLOGY
Pathology is scientific study of structure and function of the body in disease. The discipline of
pathology forms a vital bridge between initial learning phase of preclinical sciences and the final
phase of clinical subjects. PATHOLOGY is the study (logos) of suffering (pathos). It is a discipline
involving both basic science and clinical practice and is devoted to the study of the structural and
functional changes in the cells, tissues, and organs that underlie “diseases”.
Pathology studies (1) cause of the disease (etiology), (2) the mechanisms of its development
(pathogenesis), (3) the structural alterations induced in the cells, organs and tissues of the body
(morphological changes), and (4) the functional consequences of the morphologic changes (clinical
significance).
CELLULAR INJURY AND CELLULAR DEATH
Etiology of cellular injury
The causes of cellular injury, reversible or irreversible, may be broadly classified into two large
groups:
1. Genetic causes.
2. Acquired causes.
The acquired causes of disease comprise the vast majority of common diseases and can be
further categorised as the follows:
1. Hypoxia and ischemia.
2. Physical agents (mechanical trauma, thermal trauma, ultraviolet and ionizing radiation, rapid
changes in atmospheric pressure).
3. Chemical agents and drugs.
4. Infectious agents.
5. Immunologic agents.
6. Nutritional derangements.
7. Physiologic factors.
Acute Cell Injury
Reversible cellular injury is characterized with the ability of the cell to return to its normal
state after withdrawal of an acute stress.
Reversible injury is manifested with hydropic swelling of the cell (cellular edema), dilation of
endoplasmic reticulum, and detachment of ribosomes from the granular endoplasmic reticulum,
dissociation of polysomes into monosomes, mitochondria swelling and enlargement, blebs of plasma
membrane, nucleolar alterations with disaggregation of granular and fibrilar elements.
Irreversible cellular injury or cellular death is necrosis and apoptosis.
Morphogenetic mechanisms of intra- and extracellular accumulations
Mechanisms of the development of intra- and extracellular (stromal) degenerations
(dystrophies) are the followings:
1. Infiltration – redundant accumulation (deposition) of metabolites into the cells and
extracellular matrix.
2. Decomposition (phanerosis) – disintegration of membranous structures of the cells and
extracellular matrix.
3. Perverted synthesis - synthesis of abnormal substances in the cells and tissues.
4. Transformation – formation of one type of metabolism’s products from common initial
substances for proteins, fats and carbohydrates.
5
INTRACELLULAR ACCUMULATIONS
(PARENCHYMAL DEGENERATIONS OR DYSTROPHIES)
Intracellular accumulations are the accumulation of abnormal amounts of various
substances in the cells. The stockpiled substances fall into three categories:
1. A normal cellular constituent accumulated in excess, such as water, lipid, protein, and
carbohydrates.
2. An abnormal substance such as mineral, or a product of abnormal metabolism.
3. A pigment or an infectious product.
Parenchymal degenerations occur in functional cells such as: cells of a liver, kidneys, a
myocardium and are characterized by accumulation in their cytoplasm proteins, fats and
carbohydrates. It is accompanied by decrease (reduction) of function of enzymic systems and
occurrence of structural changes in cells. The most often causes of parenchymal dystrophies are
hypoxia, the intoxication, and also enzymopathy - genetically determined diseases at which is
observed an inconsistency of enzymic systems in cells. In result enzymopathy there is an
accumulation in cells of any products of a metabolism. Such diseases are named as storage diseases.
Intracellular proteinous degenerations
There are four kinds of intracellular accumulations of proteins:
1. A granular degeneration (dystrophy). Macroscopical kind of organs at this dystrophy
it is determined as “muddy or dim swelling”. At a section the organs are dim, swollen. Microscopical
descriptions of cells on electronics level: presence of electrondense granules in cytoplasm of the cells.
2. Hyaline-drop degeneration (dystrophy) is characterized by the aggregation of small
proteins granules into cytoplasm of cells. It is not determined macroscopically. This dystrophy occurs
in kidneys, liver and myocardium. The cytoplasm of plasma cells shows pink hyaline inclusions called
Russell's bodies representing synthesised immunoglobulins, the cytoplasm of hepatocytes shows
eosinophilic globular deposits of a mutant protein. Mallory's body or alcoholic hyaline in the
hepatocytes is intracellular accumulation of intermediate filaments of cytokeratin. The outcome is
negative. The focal or total coagulative necrosis develops.
3. Hydropic (cloudy, vacuolar, balloon) degeneration is characterized by
accumulation of water within the cell due to cytoplasmic vacuolation. The common causes include
bacterial toxins, chemicals, poisons, burns, and high fever. The affected organ such as kidney, liver or
heart muscle is enlarged. The cut surface bulges outwards and is slightly opaque. Microscopically: the
cells are swollen and the microvasculature compressed. Small clear vacuoles are seen in the cells.
These vacuoles represent distended cisternas of the endoplasmic reticulum. Ultrastructural changes in
hydropic swelling include the following:
Dilation of endoplasmic reticulum.
Mitochondrial swelling.
Blebs on the plasma membrane.
Loss of fibrillanty of nucleolus.
The outcome is negative, because the focal or total colliquative cellular necrosis develops.
4. Keratoid (horney) degeneration is characterized by increase production of keratin
substance. This process may be local and general. The intracellular keratin may be located in
epidermis of skin, keratinic squamous epithelial cells, cervix, and esophagus. Leucoplakia means
hyperkeratosis in mucosa. Leucoplakia may lead to malignization. For example: Squamous cell
carcinoma with keratinization. The groups of keratinized cells can be found in the center of squamous
cell carcinoma’s areas. These cell’s complexes here and there look like rose color homogenous found
forms (“canceromatous perls”).
Intracellular fatty degenerations
Intracellular fatty degenerations are the abnormal accumulations of triglycerides within
parenchymal cells. The heart, liver, kidneys are damaged most frequently.
The main cause of fatty degeneration is hypoxia, which may be due to:
1. Excess alcohol consumption (most commonly).
2. Chronic cardiovascular and chronic pulmonary insufficiency.
3. Cachexia, avitaminosis.
4. Infections (e.g. diphtheria, tuberculosis).
5. Late period of pregnancy.
6. Starvation.
6
7. Malnutrition.
8. Hepatotoxins (e. g. carbon tetrachloride, chloroform).
9. Certain drugs (e. g. administration of estrogen, steroids, tetracycline).
In the case of cell injury by chronic alcoholism, many factors are implicated with increased
lipolysis, increased free fatty acid synthesis, decreased tryglyceride utilisation, decreased fatty acid
oxidation to ketone bodies, and block in lipoprotein excretion.
An alcoholic who has not developed progressive fibrosis in the form of cirrhosis, the enlarged
fatty liver may return to normal if the person becomes teetotaler.
Morphological features of fatty change:
Fat in the tissue can be demonstrated by frozen section followed by fat stains such as Sudan 3
(red color), oil red O and osmic acid.
1. Fatty degeneration of the liver
Macroscopically the fatty liver is enlarged with rounded margins.
The cut surface bulges slightly and is pale-yellow and is greasy to touch. It is called “goose
liver”.
Microscopically: there are numerous lipid vacuoles in the cytoplasm of hepatocytes. The
vacuoles are initially small (microvesicular), but with progression of the process, the
vacuoles become larger pushing the nucleus to the periphery of the cells (macrovesicular).
At times, the hepatocytes laden with large lipid vacuoles may rupture and lipid vacuoles
coalesce to form fatty cysts. Infrequently, lipogranulomas may appear.
2. Fatty degeneration of the heart
It is also called “Tiger’s” heart.
Macroscopically the heart is enlarged, the chambers are stretched, flabby.
Microscopically we can see dust-like fatty vacuoles in the cardiomyocytes.
It is observed in the papillary muscles and trabecules of the ventricles in the form of bands
(surrounding the veins).
3. The kidneys look like “large white kidney”. They are enlarged, flabby. The cortical
substance is gray with yellow drops.
Outcomes of fatty degenerations are seldom reversible. Necrosis or sclerosis may develop.
Intracellular carbohydrate degenerations
Carbohydrates are divided into 3 groups:
1. Polysaccharides (glycogen).
2. Mucopolysaccharides.
3. Glycoproteides (mucin, mucoid).
There are several special reactions for identification of these carbohydrates. Best’s carmine and
PAS (periodic acid-Schiff) staining may be employed to confirm the presence of glycogen in cells.
Polysaccharides and mucopolysaccarides are stained dark pink or red. Staining according to Haile for identification glycoproteides. Glycoproteides are stained blue.
Accumulations of glycogen
Accumulations of glycogen are excessive intracellular deposits of glycogen usually in
patients with an abnormality of either glucose or glycogen metabolism.
Morphological features – appearance of glycogen masses as clear vacuoles within the
cytoplasm developed with special stain – PAS-reaction.
In diabetes mellitus - the prime example of this disorder – the red color granules of
glycogen can be found with large magnification in the epithelial cells of Henley’s loops and
in the lumen of kidney’s canals.
Amount of glycogen in the tissues reduces sharply (e.g. in the liver) which causes its fat
infiltration (fatty liver degeneration).
Mucoid change
Mucus secreted by mucous glands is a combination of proteins complexes with
mucopolysaccharides Mucin, a glycoprotein, is its main constituent. Mucin is normally produced by
epithelial cells of mucous membranes and mucous glands, as well as by some connective tissues like in
the umbilical cord. Epithelial mucin is stained positively with periodic acid-Shiff (PAS), while
connective tissue mucin does not but is stained positively with colloidal iron. Both are, however,
stained by alcian blue.
Epithelial mucin is associated with:
7
Catarrhal inflammation of mucous membrane (e. g. of respiratory tract, alimentary tract,
uterus).
Obstruction of duct leading to mucocele in the oral cavity, chronic appendicitis and gall
bladder.
Cystic fibrosis of the pancreas or mucoviscidosis.
Mucin-secreting tumors (e. g. of ovary, stomach, large bowel etc.).
Storage diseases
There are a lot of diseases, which are due to hereditary factors and connected with metabolism
disturbance. These diseases are called storage diseases or enzymopathy.
A few general comments can be made about all storage diseases:
All the storage diseases occur as a result of autosomal recessive, or sex-(X-) linked recessive
genetic transmission.
Most of the storage diseases are lysosomal storage diseases. Out of the glycogen storage
diseases, only type II (Pompe’s disease) is due to lysosomal enzyme deficiency.
According to the type of metabolism disturbance storage diseases have been classified into:
Proteinoses
Lipidosis
Glucogenoses
The type of proteinoses, lipidosis and glycogenoses depends on the defect in the enzyme. The
most frequent lipidosis are Gaucher’s disease, Niemann-Pick disease.
Gaucher’s Disease
This is an autosomal recessive disorder in which there is deficiency of lysosomal enzyme,
glucocerebrosidase, which normally cleaves glucose from ceramide. This results in lysosomal
accumulation of glucocerebroside (ceramide-glucose) in phagocytes of the body and sometimes in the
neurons. The main sources of glucocerebroside in phagocytic cells of the body and sometimes in the
neurons are the membrane glycolipids of old leukocytes and erythrocytes, while the deposits in the
neurons consist of gangliosides.
Clinically, there are 3 types of Gaucher’s disease:
1. Type 1 or classic form is the adult form of the disease in which there is storage of glycocerebrosides
in the phagocytes of the body, principally involving the spleen, liver, bone marrow and lymph nodes.
This is the most common type comprising 80% of all cases of Gaucher’s disease.
2. Type II is the infantile form in which there is progressive involvement of the central nervous
system.
3. Type III is the juvenile form of the disease having features in between type I and type II, i.e. they
have systemic involvement like in type I and progressive involvement of the central nervous system
(CNS) as in type II.
Morphology
In addition to involvement of different organs and systems (splenomegaly, hepatomegaly,
lymphadenopathy, bone marrow and cerebral involvement), a few other features include
pancytopenia, or thrombocytopenia secondary to hypersplenism, bone pains and pathologic
fractures.
Microscopically large number of characteristically distended and enlarged macrophages
called Gaucher cells which are found in the spleen, liver, bone marrow and lymph nodes,
and in the case of neuronal involvement, in the Virchow-Robin space. The cytoplasm of
these cells is abundant, granular and fibrillar resembling crumpled tissue paper. They have
mostly a single nucleus but occasionally may have two or three nuclei. These cells often
show erythrophagocytosis and are rich in acid phosphatase.
Niemann-Pick Disease
This is also an autosomal recessive disorder characterized by accumulation of
sphingomyelin and cholesterol.
Majority of the cases (about 80%) have deficiency of sphingomyelinase, which is required
for cleavage of sphingomyelin, while a few cases probably result from deficiency of an
activator protein.
The condition presents in infancy and is characterized by hepatosplenomegaly,
lymphadenopathy and physical and mental underdevelopment.
8
About a quarter of patients present with familial amaurotic idiocy with characteristic
cherry-red spots in the macula of the retina.
The storage of sphingomyelin and cholesterol occurs within the lysosomes, particularly in
the cells of mononuclear phagocyte system.
The cells of Niemann-Pick disease are somewhat smaller than Gaucher cells and their
cytoplasm is not wrinkled but is instead foamy and vacuolated which stains positively with
fat stains.
These cells are located in the spleen, liver, lymph nodes, bone marrow, lungs, intestine and
brain.
The most frequent glycogen storage diseases or glycogenosis are Pompe’s disease, Mc Ardle’s
disease and Gierke disease. There is defective metabolism of glycogen due to genetic disorders.
Pompe’s Disease. This is also an autosomal recessive disorder due to deficiency of a
lysosomal enzyme, acid mahase. Its deficiency results in accumulation of glycogen in many tissues,
most often in the heart and skeletal muscles leading to cardiomegaly and hypotonia.
Mc Ardle’s Disease. The condition occurs due to deficiency of muscle phosphorylase
resulting in accumulation of glycogen in the muscle (deficiency of liver phosphorylase). The disease is
common in 2nd to 4th decades of life and is characterized by painful muscle cramps, especially after
exercise, and detection of myoglobinuria in half the cases.
Gierke Disease. This condition is inherited as an autosomal recessive disorder due to
deficiency of enzyme, glucose-6-phosphatase. In the absence of glucose-6-phosphatase, excess of
normal type of glycogen accumulates in the liver and also results in hypoglycemia due to reduced
formation of free glucose from glycogen. As results, fat is metabolized for energy requirement leading
to hyperlipoproteinemia and ketosis. Other changes due to deranged glucose metabolism are
hyperuricemia. The disease manifests clinically in infancy with failure to thrive and stunted growth.
Most prominent feature is enormous hepatomegaly with intracytoplasmic and intranuclear glycogen.
The kidneys are also enlarged and show intracytoplasmic glycogen in tubular epithelial cells. Other
features include gout, skin xanthomas and bleeding tendencies due to platelet dysfunction.
The outcome of storage diseases is unfavorable because of insufficienty of the respective organ.
EXTRACELLULAR ACCUMULATIONS
(MESENCHYMAL DEGENERATIONS)
Mescnchymal (stromal vascular) degenerations develop in the connective tissue as a result of
metabolic disturbances in it.
Stromal vascular proteinous degenerations
Proteinous mesenchymal degenerations occur as mucoid swelling, fibrinoid changes,
hyalinosis and amyloidosis.
The first three types are the stages of connective tissue disorganization. The causes of mucoid
swelling, fibrinous changes and hyalinosis are the same as they are the stages of one process. They are
immunopathological and autoimmune states, hypoxia, infections. These types of connective tissue
disorganization are frequently observed in hypertension, rheumatism and other diseases of the
connective tissue accompanied by immune disturbances as well as in allergic diseases, diabetes
mellitus, etc. In the majority of cases the arterial walls, heart valves, endocardium, epicardium,
articular connective tissue are involved.
1. Mucoid swelling
Mucoid swelling is superficial reversible disorganization of the connective tissue.
These processes are associated with swelling of collagen fibers, increased vascular
permeability (due to glucosaminoglycans (GAG) action) and plasmorrhagia.
Microscopic examination shows metachromasia. Under normal conditions the main
substance is basophilic. In this case staining with toluidine blue demonstrates reddish
coloring.
Macroscopic appearance is absent.
The outcome may be reversible. In other cases, the development of fibrinoid swelling is
possible.
2. Fibrinoid changes
Fibrinoid swelling is deep irreversible connective tissue disorganization.
9
Fibrinoid is formed as a result of the main substance destruction and more increase in
vascular permeability.
The appearance of the organs is changed a little.
The main signs are revealed microscopically: the bands of collagen fibers are homogenous,
impregnated with plasma proteins.
Metachromasia is not marked due to GAG depolymerization of the main substance.
Fibrinoid swelling may be generalized (in systemic diseases of the connective tissue) and
localized (in chronic inflammations).
The outcomes are fibrinoid necrosis, sclerosis or hyalinosis.
3. Hyaline changes (hyalinosis)
Hyaline changes (hyalinosis) - (greek “hyalos” - transparent, glass-like) usually refers to an
alteration within cells or in the extracellular matrix, which gives a homogenous, glassy, pink
appearance in routine histologic sections stained with hematoxilin and eosin.
Hyalinosis develops as a result of plasma infiltration, fibrinoid swelling, inflammation,
necrosis, and sclerosis.
Hyalinosis is classified according to its localization (vascular hyalinosis and connective
tissue hyalinosis) and propagation (generalized and localized).
Vascular hyalinosis involves the arterioles and small arteries. In their walls, the
endothelium, basement membranes, and smooth muscle cells are damaged.
Three types of vascular hyaline are distinguished depending on the pathogenetie character
of its formation: 1) simple, 2) lipohyaline, 3) compound hyaline.
Microscopic study of the arteries demonstrates thickened walls with sharply narrowed or
obliterated lumen. At first, hyaline is accumulated in subendothelial areas of the vascular
wall, and then it destroys elastic and middle membranes.
In long-standing hypertension and diabetes mellitus, the walls of arterioles, especially in
the kidney, become hyalinized, owing to extravasated plasma’s protein and deposition of
basement membrane material.
Hyalinosis of connective tissue is usually localized; it develops in scars, adhesions, in the
areas of chronic inflammation (e.g. “glazed spleen”).
The outcome of hyalinosis is irreversible.
Functional significance of hyalin is different. Thus, vascular hyalinosis may lead to atrophy
or sclerosis, infarction of organs. Local hyalinosis in the cardiac valves results in heart
defects.
Amyloidosis
Amyloidosis is the term used for a group of diseases characterised by extracellular deposition
of fibrillar proteinaceous substance called amyloid.
Nature and etiology
Amyloid is composed of 2 main types of complex proteins:
1. Fibril proteins comprising about 90% of amyloid.
2. P-component constituting the remaining 10% of amyloid.
Fibril Proteins
By electron microscopy the major component of amyloid material (about 90%) consists of
meshwork of fibril proteins. Chemically 2 major forms of amyloid fibril proteins are identified which
have different origins and are seen in distinct clinicopathologic conditions:
AL (amyloid light chain) protein. AL protein of fibrils consists of polypeptides, which may
be made up of whole immunoglobulm light chains or fragment of light chains. AL type of
fibril protein is produced by immunoglobulin-secreting cells and is, therefore, seen in
association with plasma cell dyscrasias. The stimulus for production of AL-amyloid is some
disorder of immunoglobulin synthesis (multiple myeloma). B-cell lymphoma, other plasma
cells dyscrasias.
AA (amyloid associated) protein. AA protein consists of polypeptides having 76 amino acids
and is derived from larger precursor protein in the serum called SAA (serum amyloidassociated protein). In the plasma SAA circulates in association with HDL3 (high-density
lipoprotein). SAA is an acute phase reactant protein synthesised in the liver, its level being
high in chronic inflammatory and traumatic conditions. It may be in chronic mflammation
and cancer, familial Mediterranean fever.
10
Other proteins. In addition a few other forms of proteins are also found in some types of
amyloid
Transthyretin (ATTR) is a serum protein that transports thyroxine and retinol normally
while a variant of transthyretin is deposited in familial amyloid polyneuropathies and in
senile amyloidosis.
A2-microglobulm (A2m) is amyloid seen in cases on long-term hemodialysis (8-10 years).
-amyloid protein (A) is distinctive from A2m and is seen in cerebral plaques as well as
cerebral blood vessels in Alzheimer’s disease.
Hormone precursor such as procalcitonin and pro-insulin (amyloid endocrine) and keratin
has also been reported in amyloid.
P-Component
The second component of amyloid is non-fibnllar P-component that constitutes about 10% of
amyloid material. It is synthesised in the liver and is present in all types of amyloid. It is a
glycoprotein resembling the normal serum ar glycoprotein and is PAS-positive
Classification of amyloidosis
A clinical-pathologic classification is widely used currently. According to this classification,
amyloidosis can be divided into 2 major categories each found in distinct clinical settings.
A. Systemic Amyloidosis
1. Primary amyloidosis.
This is one of the two types of systemic or generalised amyloidosis.
Primary amyloidosis associates with plasma cell dyscrasias and conteins AL-protein.
In the 25% to 40% of these cases, primary amyloidosis is the high binger of frank plasma
cell neoplasia, such as multiple myeloma or other B-cell lymphomas.
Primary amyloidosis is often severe in the heart, bowel, skin, skeletal muscle, and less often
in the solid abdominal viscera.
This type of amyloidosis is most common form in the world.
2. Secondary (reactive) amyloidosis.
The second form of systemic or generalised amyloidosis is reactive or secondary in which
the fibril proteins contain AA amyloid.
Secondary or reactive amyloidosis occurs as a complication of chronic infectious or
noninfectious inflammatory conditions associated with tissues destruction such as
tuberculosis, bronchiectasis, chronic osteomyelitis, chronic pyelonephritis, leprosy,
autoimmune disorders (rheumatoid arthritis, dermatomyositis and scleroderma),
inflammatory bowel disease (ulcerative colitis and Crohn’s disease) and some tumors (renal
cell carcinoma and Hodgkin’s disease).
Secondary amyloidosis is typically distributed in solid abdominal viscera like the liver,
spleen, kidneys and adrenals Secondary reactive amyloidosis is seen less frequently in
developed countries due to containment of infections before they become chronic, but this
is the most common type of amyloidosis in underdeveloped and developing countries of the
world.
3. Familial amyloidosis.
Familial amyloidosis is seen in patients with familial Mediterranean fever and familial
amyloidotic polyneuropathy.
Familial Mediterranean fever is an autosomal recessive disease. The condition is
characterised by periodic attacks of fever and polyserositis.
Amyloidosis occurring in these cases is AA type.
Hereditary polyneuropathic amyloidosis is an autosomal dominant disorder in which
amyloid is deposited in the peripheral and autonomic nerves.
B. Localized Amyloidosis
Senile cardiac amyloidosis is seen in 50% of people above the age of 70 years. The deposits
are seen in the heart and aorta.
Senile cerebral amyloidosis is deposition of amyloid material in the walls of cerebral blood
vessels in 60% of people above the age of 70 years. Patients of Alzheimer’s disease also
develop amyloid in the senile plaques.
Endocrine amyloidosis. Some endocrine tumors are associated with microscopic deposits of
amyloid in medullary carcinoma of the thyroid, and islet cell tumor of the pancreas.
Morphology
11
Macroscopically, the affected organs are often enlarged and firm and have a waxy appearance.
If the deposits are sufficiently large, painting the cut surface with iodine imparts a yellow color that is
transformed to blue violet after application of sulfuric acid.
The histologic diagnosis of amyloid is based almost entirely on its staining characteristics:
H & E. Amyloid by light microscopy with haematoxylin and eosin staining appears as
extracellular, homogeneous, structureless and eosinophilic hyaline material
Metachromatic stains (Rosaniline Dyes). Amyloid has the property of metachromasia, i.
e. the dye reacts with amyloid and undergoes a color change. Metachromatic stains
employed are rosaniline dyes such as methyl-violet and crystal-violet, which impart rosepink coloration to amyloid deposits.
Congo red. All types of amyloid have affinity for Congo red stain. The stain may be used on
both gross specimens and microscopic sections amyloid stains an orange color. The stain
can also be used to distinguish between AL and AA amyloid (primary and secondary
amyloid respectively). After prior treatment with permangnate on the section, Congo red
stain is repeated: in the case of primary amyloid (AL amyloid), the Congo red positivity
(congophilia) persists while it turns negative for Congo red in secondary amyloid (AA
amyloid).
Sulfated alcian blue. This is a nonspecitic screening test and imparts blue-green color to
amyloid positive areas.
lmmunohistochemistry. More recently, immunohistochemical stains can classify type of
amyloid. Antibody specific for fibril protein gives positive immunoreactivity.
Diagnosis of amyloidosis
Histologic examination of biopsy material is the commonest and confirmatory method for
diagnosis in a suspected case of amyloidosis. If renal manifestations are present, kidney is the
preferred site for biopsy. Otherwise the commonly accessible sites such as rectum, gingiva, and more
recently abdominal fat, are biopsied and are followed by Congo red staining for confirmation.
Pathologic changes in organs
Amyloidosis of Kidneys
Amyloidosis of the kidneys is most common and most serious because of ill-effects on renal
function.
The deposits in the kidneys are found in most cases of secondary amyloidosis and in about
one third cases of primary amyloidosis.
The kidneys may be normal-sized, enlarged or terminally contracted due to ischemic effect
of narrowing of vascular lumina. Cut surface is pale waxy and translucent.
Amyloid deposition occurs primarily in the glomeruli though it may involve peritubular
interstitial tissue and the walls of arterioles as well:
a) In the glomeruli, the deposits initially appear on the basement membrane of the
glomerular capillaries, but later extend to produce luminal narrowing and distortion of
the glomerular capillary tuft.
b) In the tubules, the amyloid deposits likewise begin close to the tubular epithelial
basement membrane.
c) The vascular involvement affects chiefly the walls of small arterioles and venules,
producing narrowing of their lumina and consequent ischemic effects.
Amyloidosis of Spleen
Two patterns are observed:
“Sago spleen”. The splenomegaly is not marked and cut surface shows characteristic
translucent pale and waxy nodules resembling sago grains and hence the name.
Microscopically, the amyloid deposits begin in the walls of the arterioles of the white pulp
and may subsequently replace the follicles.
“Lardaceous spleen”. There is generally moderate to marked splenomegaly (weight up
to 1 kg). Cut surface of the spleen shows map-like areas of amyloid. Microscopically, the
deposits involve the walls of splenic sinuses and the small arteries and in the connective
tissue of the red pulp.
Amyloidosis of Liver. The liver is often enlarged pale, waxy and firm. The amyloid initially
appears in the space of Disse, but later as it increases; it compresses the cords of hepatocytes.
Amyloidosis of Heart.
12
Heart is involved in systemic amyloidosis quite commonly more so in the primary than in
secondary systemic amyloidosis. It may also be involved in localised form of amyloidosis in
very old patients.
Amyloidosis of the heart may produce arrhythmias due to deposition in the conduction’s
system. The heart shows tiny nodular deposits of amyioid underneath the endocardium.
Later, there may be a pressure atrophy and impaired ventricular function, which may
produce restrictive cardiomyopathy.
Amyloidosis of Alimentary tract. Involvement of the gastrointestinal tract by amyloidosis
may occur at any level from the oral cavity to the anus. Rectal and gingival biopsies are the common
sites for diagnosis of systemic amyloidosis.
The prognosis for patients with generalized amyloidosis is poor. Those with immunocytederived amyloidosis have a median survival of 2 years after diagnosis.
Stromal vascular fatty degenerations
Stromal fatty infiltration is the deposition of mature adipose cells in the stromal connective
tissue. The condition occurs most often in patients with obesity.
As a rule it is a generalized process when the amount of fat in the depots increases.
Depending on the excess of the patient mass compared to the norm, 4 degrees of
obesity are defined:
1. If the patient’s mass increases by 20 -29% we distinguish 1st degree of obesity;
2. If the patient's mass increases by 30 -49% - 2nd degree;
3. If the patient's mass increases by 50 - 99% - 3rd degree;
4. If the patient's mass increases by 100% and more 4th degree of obesity.
The two commonly affected organs are the heart and the pancreas.
The damage to these organs is most serious.
Subepicardial fat covers the heart as a case, invades the myocardial stroma causing atrophy
and sclerosis.
If the connective tissue does not grow, heart rupture in the area of fat growth may occur.
In pancreatic lipomatosis beta-cell atrophy and diabetes mellitus are possible.
According to the etiology the following types of obesity are defined:
1. Primary (idiopathic);
2. Secondary.
There are several types of secondary obesity:
1. Alimentary.
2. Cerebral.
3. Endocrine.
4. Hereditary in Gierke’s disease.
According to the patient's appearance, obesity may be
1. Symmetrical
2. Upper
3. Medial
4. Lower.
According to morphological peculiarities of adipose tissue, it may be:
1. Hypertrophic.
2. Hyperplastic.
In hypertrophic type adipose tissue enlarges due to increased volume of fatty cells, in
hyperplastic due to increase in their number. Obesity is a severe complication of mainly endocrine and
nervous diseases. Alimentary obesity is also unfavorable for the organism. As a rule such patients
develop ischemic heart disease.
Local enlargement of adipose tissue (lipomatosis) occurs in Dercum's disease when painful fat
nodes appear in the subcutaneous fat of the lower and upper extremities and trunk.
Sharp reduction in the amount of neutral fat in the whole organism is called cachexia.
Disturbance in cholesterol and its esters metabolism causes atherosclerosis. The wall
of the vessel is thicken everywhere, but much more it is thicken because of the formation of the
atherosclerotic plaque, which are composed with lipids and fibrotic tissue.
Stromal vascular carbohydrate degenerations
Stromal vascular carbohydrate degenerations develop due to disturbance of
glycosaminoglycans and glycoproteids metabolism. When glycoproteid metabolism is disturbed,
13
chromotropic substances are released from the protein bonds. They accumulate in the main substance
of the connective tissue. Collagen fibers change into mucus-like mass.
Connective tissue mucin is associated with:
Mucoid or myxoid degeneration in some tumors (myxomas).
Neurofibromas, soft tissue sarcomas etc.
Myxomatous change in the dermis in myxedema.
Myxoid change in the synovium in ganglion on the wrist.
The condition results in colliquative necrosis with formation of cavities filled with mucus.
Mucopolysaccharidoses (MPS)
Disturbance of glycosaminoglycans (GAG) is due to hereditary factors as in a storage
disease.
It is characterized by deficiency of specific lysosomal enzyme involved in the degradation of
mucopolysaccharides or glycosaminoglycans.
Syndrome of MPS manifests in infancy or early childhood and involves multiple organs and
tissues, chiefly connective tissues, liver, spleen, bone marrow, lymph nodes, kidneys, heart
and brain.
The mucopolysaccarides accumulate in mononuclear phagocytic cells, endothelial cells,
smooth muscle cells and fibroblasts.
The material is finely granular and PAS-positive by light microscopy.
By electron microscopy, it appears in the swollen lysosomes and can be identified
biochemically as mucopolysaccharide.
The most frequent of them are Pfaundler-Hurler disease, or gargoilism. Its cause is
congenital defect of the enzyme determining GAG metabolism. This disease is characterized
by irregular skeleton growth, “massive” skull, heart defects, inguinal and umbilical hernias,
hepato- and splenomegaly, keratoleukoma (retina opacity).
PATHOLOGY OF PIGMENTS
Pigments are colored substances, some of which are normal constituents of cells where as
others are abnormal and collect in cells only under special circumstances.
Pigments are generally classified into two broad categories:
Endogenous pigments, which are normal constituents of cells and tissues;
Exogenous pigments introduced into the body from environment.
Classification of endogenous pigments
1. Hemoglobinogenic pigments.
2. Proteinigenic.
3. Lipidogenic.
Pigments derived from hemoproteins appear as a result of physiologic destruction of
erythrocytes.
Physiologic pigments
1. Ferritin is a ferroproteide. It is located in liver, spleen, bone marrow and lymphatic nodes.
2. Hemosiderin is iron-containing pigment. Hemosiderin, which is formed by aggregates of ferritin
and is identifiable by light microscopy as golden-yellow to brown, granular pigment, in the
mononuclear phagocytes of the bone marrow, spleen and liver. Hemosiderin is ferric iron that can be
demonstrated by Prussian blue reaction
3. Bilirubin is iron-free pigment.
Pathologic pigments
1. Hematoidin is iron-free, orange-brown crystal pigment. It’s formed extravascularly in the center of
hemorrhages or foci of necrosis at anaerobic conditions.
2. Hematin is a brown-black pigment derived from hemoglobin and has 2 types:
Chloric hematin is formed in gastric erosions and ulcers as a result of interaction between
hemoglobin and gastric excretion (muriate acid).
Hemomelanin is a brown pigment produced by malarial parasites from hemoglobin; it’s
taken up by monocytes in the blood and subsequently deposited in the liver and spleen.
3. Porfirin is precursor of hem. It deposits in blood and urine. Clinical symptoms are photophobia,
erythema, and dermatitis. Spleen, bones, teeth, urine becomes of dark red. Porphyria develops when
porphyrin metabolism is disturbed. It may be congenital and acquired. Acquired porphyria is
14
observed in intoxications, avitaminosis (pellagra), pernicious anemia, and diseases of the liver.
Pathology of hemosiderin’s metabolism
Hemosiderosis
Hemosiderosis occurs in two situations:
Local hemosiderosis.
It is characterized by local breakdown of red cells in tissues, e.g. in internal hemorrhage.
Mechanism of local hemosiderosis is extravascular hemolysis.
It occurs regularly around areas of bruising and hemorrhage.
In each instance the pigment is localized in cells of the reticuloendothelial system.
In the lungs hemosiderin-laden macrophages (siderofages) are appropriately referred to as
“heart failure cells”.
Visceral siderosis (systemic hemosiderosis).
Mechanism of systemic hemosiderosis is intravascular hemolysis.
It is seen in the liver, spleen and sometimes in kidneys in cases of hemolytic anemia, and in
patients requiring repeated blood transfusion. The generalized form of this condition also
referred to as secondary hemochromatosis.
The pigment imparts a deep brown color to tissues and organs when it is present in high
concentrations.
It can also occur in patients with chronic ineffective erythropoiesis (such as thalassemia
major).
Alcohol ingestion when carried to extremes can lead to hemosiderosis because of the
augmentation of iron uptake by alcohol.
In hemochromatosis, a genetic disorder, the absorbtion of iron is virtually uncontrolled.
The system becomes overload and iron is deposited as hemosiderin in many sites, the main
ones being:
- Pancreas – associated with fibrosis, which may destroy islet tissue (diabetes mellitus).
- Liver – usually associated with fibrosis (cirrhosis).
- Skin – mainly around swet glands. Excessive melanin is also deposited; hence this
condition is sometimes termed “bronzed diabetes”.
- Heart musle.
- Mesenteric lymph nodes.
Pathology of bilirubin’s metabolism
Jaundice
When the bilirubin content of the serum rises above 34 mmol/l, jaundice appears.
Types of jaundice
1. Prehepatic jaundice (Hemolytic jaundice) - results from an excessive breakdown of the red blood
cell membrane in a variety of conditions, which include:
A genetic membrane defect.
An immune reaction.
Circulating of intravascular toxic substances causing red cell destruction (snake poison).
Hemolytic (familial) jaundice in spherocytosis.
Sickle cell anemia.
Hemolytic disease of the newborn, Rh incompatibility.
Incompatible blood transfusion.
Infections (malaria, clostridial infection, mycoplasma pneumonia, sepsis).
Leukemia.
In these conditions the excessive amount of pigment has not pass through the liver for
conjugation. The liver’s capacity to conjugate it is exceeded, and the level of unconjugated bilirubin
rises in the plasma. It can crystallize out in the tissues, in the brain, may cause necrosis. Injury of
brain may lead to bilirubin encephalopathy (kernicterus).
2. Intrahepatic jaundice (Hepatocellular jaundice) - results from failure both of hepatocytes to
conjugate bilirubin and of bilirubin to pass through the liver into the intestine. Both of conjugated
bilirubin and unconjugated bilirubin increase its amount in blood. The liver is light yellowish-green
color of saffron (“saffron liver”).
Failure of conjugation may involve:
Hepatocellular jaundice, e.g., viral hepatitis and hypoxic necrosis.
15
Drug-induced jaundice, e.g., disturbance of glucoronide conjugation.
Intrahepatic cholestasis, e.g., congenital intrahepatic occlusion, tumors, inflammations,
or cirrhosis.
Mushroom, arsenic, phosphorous poisoning.
3. Posthepatic jaundice (Obstructive jaundice) - results from an obstruction of the passage of
conjugated bilirubin from hepatocytes to the intestine. Conjugated bilirubin is water-soluble and is
excreted in the urine. The liver is dark green.
Obstructive jaundice may appear in the following causes:
Stenosis of extrahepatic bile ducts.
Gall stones in the major ducts.
Pancreatic tumor compression.
Fibrosis involving the small intrahepatic ducts; the bile ducts became distended with
conjugated bilirubin, which is reabsorbed.
In the liver, bile pigments may appear:
a) As bile pigment droplets in the hepatocytes.
b) As bile impregnations in necrotic areas.
c) As bile casts (bile capillaries, cholangioles, or bile canaliculi).
d) In Kupffer’s cells.
Pathology of the metabolism of proteinogenic pigments
Melanin is a normal pigment found in the form of fine brown granules in the skin, choroids
of the eye, adrenal medulla, and hair and sometimes in the meninges and intestine.
Melanin is a brown-black pigment synthesized by melanocytes from tyrosine by its
oxidation.
After secretion of the pigment, it’s taken up by adjacent epidermal cells and phagocytic
melanophores in the underlying dermis
Ultraviolet radiation stimulates the synthesis of melanin.
Various disorders of melanin pigmentation cause generalized and localized
hyperpigmentation and hypopigmentation.
Focal hyperpigmentation: malignant melanoma, nevus, melanosis coli, lentigo.
Nevus is a benign tumor.
Malignant melanoma is a highly malignant neoplasm that invades normal tissues early and
widely and that almost invariably terminates in death.
A dysontogenetic malformation (hamartoma) consisting of nevus cells. It’s frequently
presented at birth and grows slowly during puberty.
May be generalized melanin pigmentations: a) Addison’s disease, b) an adrenocortical
insufficiency resulting from destruction of the adrenal cortex, c) chloasma observed during
pregnancy, d) chronic arsenical poisoning.
Localised hypopigmentation: a) leucoderma is a partial albinism and is an inherited
disorder; b) vitiligo is hereditary local hypopigmentation of the skin, c) acquired focal
hypopigmentation from various causes such as leprosy, healing of wounds, syphilis,
radiation dermatitis, etc.
Albinism is an inherited generalized disorder of melanin metabolism in which there is a
decrease or absence of the pigment in the skin and choroid of the eye. Albinos have blond
hair, poor vision and severe photophobia. They are highly sensitive to sunlight.
Pathology of the metabolism of lipidogenic pigments
Lipopigments usually include lipofuscin and lipochrom. Lipofuscin is an insoluble lipid
pigment presented in cells of elderly persons and those with mulnutrition or a chronic
wasting disease.
It is a brown intracellular pigment found in hepatocytes, cardiocytes, and neurons.
Organs containing large amounts of lipofuscin are deep brown; in the heart, this is referred
as brown atrophy.
Exogenous pigments
Inhaled pigments. The most commonly inhaled substances are carbon or coal dust; others
are silica or stone dust, iron or iron oxide, asbestos and various other organic substances. Anthracosis
(i. e. deposition of carbon particles) is seen in almost every adult lung and generally provokes no
reaction of tissue injury.
16
Ingested pigments. Chronic ingestion of certain metals may produce pigmentation. Argyna
is chronic ingestion of silver compounds. Chronic lead poisoning may produce the characteristic blue
lines on teeth. Carotenemia is yellowish-red coloration of the skin caused by excessive ingestion of
carrots.
Ingested pigments (tattooing). Pigments like India ink, cinnabar and carbon are
introduced into the dermis in the process of tattooing where the pigment is taken up by macrophages
and lies permanently in the connective tissue.
Mineral metabolism disturbance
Minerals play an active role in metabolic processes of the human organism. They are
components of structural elements of cells, enzymes, hormones, vitamins, and pigments.
The most frequent disturbances in medical practice are in the metabolism of calcium, copper,
potassium, and iron.
Calcium metabolism disturbances
I. Dystrophic calcification. Dystrophic calcification refers to the macroscopic deposition of
calcium salts in injuried tissues and does not simply reflect an accumulation of calcium derived from
the bodies of dead cells. It is often visible to the naked eye, and ranges from gritty, sandlike grains to
firm, rock-hard material. Staining with H&E demonstrates calcium salts as deeply basophilic,
irregular and granular clumps. For identification of calcium salts we usually use special reaction called
silver impregnation method or Kossa’s method. Calcium deposits are stained black.
It may occur in crucial locations, such as:
1. Necrotic tissue, which is not absorbed:
Old caseous lesions of tuberculosis.
Old infarcts.
Old collections of pus.
Dead parasites (echinococci).
Old thrombi.
Dead fetus (lythopedion).
2. Tissues undergoing slow degeneration:
Hyaline areas in simple tumors.
Tissues in old age, especially fibrous tissue, cartilage, in the mitral or aortic valves after
rheumatic fever with formation of mitral or aortic stenosis or as in atherosclerotic
coronary arteries with narrowing of those vessels.
II. Metastatic calcinosis (calcium metastases) reflects deranged calcium metabolism
associated with increased serum calcium concentration (hypercalcemia). It has systemic character; its
main cause is hypercalcemia, which may be of endocrine origin in hyperproduction of Parathormone
or hypoproduction of Calcitonine. Calcium salts precipitate in different organs, more frequently in the
lungs, gastric mucosa, kidneys, myocardium, arterial walls. It may be associated with:
Reduction of calcium excretion from the organism.
Multiple fractures of the bones.
Hyperparathyroidism.
Chronic renal failure.
Multiple myeloma.
Osteomalacia (when the bone becomes soft).
Lesions of the large intestine (the place of Ca excretion).
Vitamin D intoxication.
The outcome is unfavorable, calcium does not resolve.
Copper metabolism disturbance
This appears in Wilson-Konovalov disease (hepatocerebral degeneration, hepatolenticular
degeneration).
It is a hereditary disease in which liver ceruloplasmin production decreases. Ceruloplasmin
is alpha2-globulin and can bind copper in the blood. As a result, copper becomes free from
unstable bonds with plasma proteins and sediments in the tissue.
Copper accumulates in the liver, brain, kidneys, cornea (in the cornea it looks like greenbrown ring on its margin of the cornea), in the pancreas, testes, etc.
The state is characterized by development of liver cirrhosis, degenerative symmetrical
changes in the brain in the area of lens nuclei, caudal body, pale globe, and cortex.
Copper blood plasma amount is decreased but is increased in the urine.
17
There are 3 forms of the disease: hepatic, lenticular, hepatolenticular.
The outcome is unfavorable.
Potassium metabolism disturbances
Increased blood (hyperpotassemia) and tissue potassium amount is observed in Addison’s
disease and is associated with the lesion of the adrenal cortex, the hormones of which regulate
electrolyte exchange.
Potassium deficiency characterizes periodic paralysis; hereditary disease for which attack of
weakness and motor paralysis are typical.
Formation of Stones
Stones or calculi are dense formations freely lying in the cavities of the organs or in the
ducts.
Their shape depends on the organs in which they are formed: round in the urinary bladder,
facet in the gallbladder (their faces are lapped to each other), branching in the kidneys.
Their surface may be either smooth or rough.
The color depends on their chemical composition: white (phosphates), yellow (urates), dark
brown or green (pigment).
On cut they may be crystalloid (radial structure), colloid (stratified structure) and colloidcrystalloid (radial-stratified).
Their chemical composition is different, i.e. biliary stones may be cholesterol, pigment,
calcium and combined, urinary - urates, phosphates, oxalates (calcium oxalate), cystin,
xantin.
Bronchial calculi consist of mucus inlayed with calcium.
Stones are most frequently formed in the bile ducts and urinary tract in cholelithiasis,
urolithiasis, in the excretory ducts of the pancreas, salivary glands, bronchi, crypts of the
tonsils, veins (phlebolith), intestine (coprolyth).
Both general and local factors are important for pathogenesis of calculus formation. General
factors are the main ones; they are acquired or hereditary disturbances of metabolism. Local factors
are congestion, inflammation. The immediate mechanism of calculus formation consists of two
processes: formation of organic matrix and salt crystallization. Each of these may be primary.
Compression with a stone may result in necroses in renal pelvis, gallbladder, bedsores,
perforations, inflammation (pyelocystitis, cholecystitis, cholangitis, etc.).
18
IRREVERSIBLE CELLULAR INJURY:
Cell death is a state of irreversible injury. It may occur in the living body as a local change (i.
e. autolysis, necrosis and apoptosis), or result in end of the life (somatic death).
Autolysis (“self-digestion”) is disintegration of the cell by its own hydrolytic enzymes
liberated from lysosomes. Autolysis can occur in the living body when it is surrounded by
inflammatory reaction (vital reaction), or may occur as postmortem change in which there is complete
absence of surrounding inflammatory response. Autolysis is rapid in some tissues rich in hydrolytic
enzymes such as in the pancreas, and gastric mucosa, intermediate in tissues like the heart, liver and
kidney, and slow in fibrous tissue.
Necrosis
Necrosis is celullar death in the living body in the disease. Necrosis is defined as focal death
along with degradation of tissue by hydrolytic enzymes liberated by cells. It is invariably accompanied
by inflammatory reaction.
Two essential changes bring about irreversible cell injury in necrosis - cell digestion by lytic
enzymes and denaturation of proteins.
Nuclear changes. The irreversibly damaged nuclei are characterized by one of the following
three features:
1. At first nucleus shrinks and becomes dense. This process is called karyopicnosis.
2. After that karyorrhexis develops. This process is characterised by rupture of nuclear membrane
and fragmentation of the nucleus. Nucleus is decomposed into small granules.
3. Also karyolysis may be developed, when the nucleus dissolves.
At electron microscopic level, in addition to the above nuclear changes, disorganization and
disintegration of the cytoplasmic organelles and severe damage of the plasma membrane are seen.
In the cytoplasm, protein denaturation and coagulation or hydration and colliquation take
place. Plasmorrhexis is characterized by decomposition of cytoplasm into clumps due to
coagulation. Then plasmolysis takes place. Plasmolysis is hydrolytic fusion of cytoplasm. Sometimes
we can observe vacuolization and calcification in the cytoplasm.
Stages of necrosis (or morphogenesis):
1. Paranecrosis - reversible changes; as a rule, reversible degeneration.
2. Necrobiosis - irreversible degenerative changes.
3. Death of cells.
4. Autolysis is the enzymic digestion of the dead cell due to effect of catalytic enzymes
derived from lysosomes.
Types of necrosis
According to the mechanisms of development:
1. Direct (from influence of mechanical, physical, chemical, and toxic factors).
2. Indirect (vascular and neurogenous).
According to the cause:
1. Traumatic.
2. Toxic.
3. Trophoneurotic.
4. Allergic.
5. Vascular or ischemic.
According to the morphological features:
1. Coagulative necrosis is associated with inhibition of lytic enzymes. Foci of
coagulative necrosis in the early stage are pale, firm, and slightly swollen. With
progression they become more yellowish, softer, and shrunken. The cells do not lyse;
thus, their outlines are relatively preserved. Nuclei disappear and the acidified
cytoplasm becomes eosiniphilic. Waxy (Zenker’s) necrosis of muscle may occur at
typhoid fever.
2. Liquefactive (colliquative) necrosis is marked by dissolution of tissue due to
enzymatic lysis of dead cells. Typically, it takes place in the brain when autocatalytic
enzymes are released from dead cells. Liquefactive necrosis occurs also in purulent
inflammation due to the heterolytic action of polymorphonuclear leucocytes in pus.
Liquefied tissue is soft, diffluent and composed of disintegrated cells and fluid.
3. Gangrene – develops in organs and tissues having contact with environment. The
most often examples of gangrene are gangrene of low extremities, uterus, lungs etc.
19
There are 3 main forms of gangrene - dry, wet and gas gangrene. Contrasting features
of two main forms of gangrene are summarised in Table 1.
Sequester – fragment of dead tissue, which can’t be autolized, replaced by connective
tissue and which is localized among alive tissue.
4. Infarction – vascular or ischemic necrosis.
5. Fat necrosis is encountered in adipose tissue contiguous to the pancreas and more
rarely at distant sites, as a result of leakage of lipase after acute injury to pancreatic
acinar tissue, most commonly from obstruction of pancreatic ducts. Grossly, fat
necrosis appears as firm, yellow-white deposits in peripancreatic and mesenteric
adipose tissue. Histologically, necrotic fat cells are distinguishable as pale outlines, and
their cytoplasm is filled with an amorphous-appearing, faintly basophilic material
(soap).
6. Caseous necrosis has features of both coagulative and liquefactive necrosis.
Typically, it occurs in the center of tuberculous granulomas, which contain a white or
yellow “cheesy” material (Latin caseum = cheese) that accounts for the name of this
lesion. Histologically, the outlines of necrotic cells are not preserved, but the tissue has
not been liquefied either. The remnants of the cells appear as finely granular,
amorphous material.
7. Fibrinoid necrosis is characterised by deposition of fibrin-like material, which has
the staining properties of fibrin. It is encountered in various examples of immunologic
tissue injury, arterioles in hypertension, peptic ulcer etc. Histologically, fibrinoid
necrosis is identified by brightly eosinophilic, hyaline like deposition in the vessel’s
wall or on the luminal surface of a peptic ulcer.
Outcomes of necrosis
Regeneration of tissues – replacement of the dead tissue with a new one.
Incapsulation – formation of the connective tissue capsula around necrotic area.
Organization – replacement of the dead tissue with connective tissue.
Petrification – replacement of the dead tissue with calcium salts.
Incrustation – replacement of the dead tissue with any other salts except calcium.
Ossification – the formation of the bone tissue in the necrotic area;
Hyaline change – the appearance of the hyaline-like substance in the necrotic area.
Suppuration or purulent fusion of necrotic tissues.
Sequestration – formation of sequester.
Mutilation – spontaneous tearing- away of the dead tissue.
Cystic formation.
Apoptosis
Apoptosis is a programmed (physiological) death of the cell in the living body.
Morphologic features of apoptosis:
1. Cell shrinkage;
2. Chromatin condensation;
3. Formation of cytoplasmic blebs and apoptotic bodies;
4. Phagocytosis of apoptotic cells or bodies.
Histologically, in tissues stained with hematoxylin and eosin, apoptotic involves single cells
or small clusters of cells.
The apoptotic cell appears as a round or oval mass of intensely eosinophilic cytoplasm with
dense nuclear chromatin fragments.
Because the cell shrinkage and formation of the apoptotic bodies are rapid, however, and
the fragments are quickly phagocytosed, degraded, or extruded into the lumen,
considerable apoptosis may occur in tissue before it becomes apparent in histologic
sections. In addition, apoptosis - in contrast to necrosis - does not elicit inflammation,
marking it even more difficulty to detect histologically.
The contrasting features of apoptosis and necrosis are summarised in Table 2.
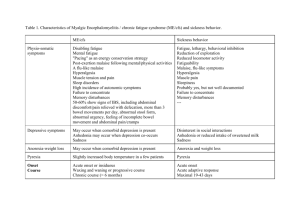
TABLE 1. Contrasting features of two main forms of gangrene
FEATURE
DRY GANGRENE
WET GANGRENE
20
Site
Commonly limbs
More common in bowel
Mechanisms
Arterial occlusion
More commonly venous obstruction
less often arterial occlusion
Macroscopy
Organ dry shrunken and black
Part moist soft swollen rotten and
dark
Putrefaction
Limited due to very little blood Marked due to stuffing of organ with
supply
blood
Line
of Present at the junct on between No clear line of demarcation
demarcation healthy and gangrenous part
Bacteria
Bacteria fail to survive
Prognosis
Generally better
septicemia
due
Numerous present
to
little Generally poor due to profound
toxemia
TABLE 2. Contrasting Features of Apoptosis and Necrosis
FEATURE
1. Definition
APOPTOSIS
NECROSIS
Programmed and coordinated cell Cell death along with degradation of
death
tissue by hydrolytic enzymes
2
Causative Physiologic
agents
processes
and
pathologic Hypoxia, toxins
3. Morphology No Inflammatory reaction
Inflammatory reaction always present
Death of single cells
Death of many adjacent cells
Cell shrinkage
Cell swelling initially
Cytoplasmic blebs on membrane
Membrane disruption
Apoptotic bodies
Damaged organelles
Chromatin condensation
Nuclear disruption
Phagocytosis of apoptotic bodies by Phagocytosis
macrophages
macrophages
of
cell
debris
by
4. Molecular Lysosomes and other organelles Lysosomal breakdown with liberation
changes
intact
of
hydrolytic
enzymes
and
oncossuppressor genes
DEATH, SIGNS OF DEATH, POSTMORTEN CHANGES
21
Death is the expression of irreversible stopping of the vital activity of organism. With
approach of death a man turns into the dead body or corpse (cadaver).
There are natural (physiologic), violent death and death after diseases.
Natural death takes place in senile persons as a result of physiologic wear of organism.
Violent death is a result of murders, suicides, traumas and accidents.
Death after diseases is a result of incompatibility of the life with changes that were
provoked by pathological (unhealthy) processes.
There are clinical and biological death:
1. Clinical death is characterized with stopping of breathing and blood circulation, which are
reversible during some minutes (the time of outliving of the brain cortex). Agony precedes clinical
death and is the result of uncoordinated actions of homeostatic systems during terminal period
(arrhythmia, paralysis of sphincters, convulsions and pulmonary edema).
2. Biological death is irreversible changes of vital activity of organism and beginning of autolytical
processes. The central nervous system dies in the fiirst 5-6 minutes. In other organs and tissues this
process lengthen out to some hours or even days.
Soon after biological death a number of signs of death and postmorten changes appears. They
are the followings:
coolness of the dead body (algor mortis) develops as the result of stopping of
warnth’s production in the body and equilization of temperature of dead body and
environment;
becoming numb of a corpse (rigor mortis) is manifested as condensation of
arbitrary and nonarbitrary muscles because of disappearing of adenosine triphosphate and
accumulation of lactic acid in them. Usually it develops in 2-5 hours after death, spreads to
all muscles of the body to the end of the first day, is kept during 2-3 days and then
disappears;
putrid drying appears because of evaporation of moisture from the surface of the body. It
may be localized or generalized (mummification). The dimness of cornea and appearance of
dark-brown patches on sclera are connected with this process;
redistribution of blood in the corpse results in repletion of veins with blood, but
arteries remain almost empty. The postmorten coagulation of veins and cavities of the right
part of the heart takes place. However in the cases of death because of asphyxia the blood
does not coagulate (asphyxia of newborns);
putrid patches appear because of redistribution of blood in the corpse and depend on its
position. The blood flows down into the veins of the lower parts of the body and
accumulates their. That’s why putrid hypostases appear in 3-6 hour after death;
putrifacation of the corpse is connected with processes of autolysis and rotting of the
corpse. Postmorten autolysis appears earlier and is more expressed in glandular organs
which cells are rich in proteolytic enzymes (liver, pancreas, stomach). Patrifacative
processes join quickly to postmorten autolysis because of proliferation of putrifactive
bacteria. Putrifacation intestifies postmorten autolysis, leading to fusion of tissues which
become to be colored in dirty-green color and exhale characteristic putrid smell. Quickness
of corpse’s aulotysis and putrifacation depends on the temperature of environment.
22
CELLULAR ADAPTATIONS
For the sake of survival on exposure to stress, the cells make adjustments with the changes in
their environment (i. e. adapt). Broadly speaking, such physiologic and pathologic adaptations occur
by
Decreasing or increasing their size (atrophy and hypertrophy respectively).
By changing the pathway of phenotypic differentiation of cells (metaplasia and dysplasia).
In general, the adaptive responses are reversible on withdrawal of stimulus.
Atrophy
Atrophy means reduction of the number and size of cells, tissues and organs in living
organism characterized by decrease or stopping their function.
Atrophy may be physiologic and pathologic.
A. Physiologic atrophy. It is a normal process of aging in some tissues:
1. Atrophy of lymphoid tissue in lymph nodes, appendix and thymus.
2. Atrophy of gonads after menopause.
3. Atrophy of brain.
4. Atrophy of bones.
It may be obliteration of the umbilical arteries and arterial duct (Botallow’s) after birth.
B. Pathologic atrophy may be general and local.
General atrophy is observed in cachexia due to
Oncologic and chronic diseases.
Starvation.
Injury of hypophysis (endocrine cachexia).
Injury of hypothalamus (cerebral cachexia).
Gross appearance of patients occurs:
Sharp exhaustion.
Adipose tissue is decreased and it has brown color.
Muscles are atrophied; skin is dry and flabby.
Internal organs are small, brown color and often shrunken.
Osteoporosis takes place.
Histologically:
Cells become smaller in size but are not dead cells.
Shrinkage in cell size is due to reduction in cell organelles.
Accumulation of lipofuscin around nucleus takes place. Lipofuscin (“wear and tear”
pigment) is a golden yellow pigment representing undigested lipid material derived from
cellular metabolism.
Local atrophy has several types:
1. Ischemic atrophy develops due to insufficiency of the blood supply. Hypoxia stimulates of the
proliferation of fibroblasts and forms sclerosis. For example: small atrophic kidney in atherosclerosis
of renal artery, atrophy of brain in cerebral atherosclerosis.
2. Disuse atrophy (dysfunctional) develops due to reduction of the function of organ: atrophy of
muscles due to immobility, atrophy of the pancreas in obstruction of pancreatic duct.
3. Neuropathic atrophy due to interrupted nerve supply: poliomyelitis, motor neuron disease, nerve
section, and inflammation of facial nerve.
4. Endocrine atrophy: hypopituitarism may lead to atrophy of thyroid, adrenal and gonads;
hypothyroidism may cause atrophy of the skin and its adnexal structures.
5. Pressure atrophy: compression of spine by tumor in nerve root, compression of skull by
meningioma arising from pia-arachnoid, compression of sternum by aneurysm of arch of aorta,
compression of renal tissue by dilated renal pelvic in hydronephrosis, compression of brain tissue by
dilated ventricles in hydrocephalus.
6. Atrophy due to chemical and physical influences. For example: action of the radiation lead to
atrophy of bone marrow and genital organs.
7. Idiopathic atrophy: myopathies, testicular atrophy.
The atrophic tissue may be replaced by fatty ingrowths. Atrophy is reversible provided the cause is
eliminated or deficiencies restored.
Hypertrophy and hyperplasia
Hypertrophy refers to an increase in the size of parenchymal resulting in enlargement of the
organ or tissue, without any change in the number of cells.
23
Hyperplasia is an increase in the number of parenchymal cells resulting in enlargement of
the organ or tissue. Quite often, both hyperplasia and hypertrophy occur together.
Mechanisms of hypertrophy
Hypertrophy of tissue arises due to increase of size of functional cells. Thus, the
hypertrophied organ has no new cells, just larger cells.
Hypertrophy of tissue arises due to increase of number of functional cells (hyperplasia of
cells).
Hypertrophy of cells arises due to both increase of size of functional cells and increase of
number of ultrastructural elements. Thus, the increased size of the cells is due not to an
increased intake of fluid, called cellular swelling or edema, but to the synthesis of more
structural components. It is called true hypotrophy.
False hypertrophy is the increase of the size of organs due to growth of connective
tissue, accumulation of the fluid or fatty tissue. It results in atrophy of organ
(hydronephrosis, hydrocephalus, obesity of heart).
True hypertrophy (hyperplasia) has adaptative and compensative characteristics and may
be physiologic and pathologic:
A. Physiologic hypertrophy (hyperplasia).
1. Neurogumoral (hormonal) hypertrophy: hypertrophy of female breast at puberty,
during pregnancy and lactation, hypertrophy of pregnant uterus, proliferative activity
of normal endometrium after a normal menstrual cycle, prostatic hyperplasia in old
age.
2. Working hypertrophy of skeletal muscle: hypertrophied muscles in athletes and
manual labour.
B. Pathologic hypertrophy (hyperplasia).
1. Neurogumoral hypertrophy develops due to impairment of endocrine functions.
Endometrial glandular hyperplasia following estrogen excess which it occurs by
metrorrhagia; atrophy of testis leads to increase of breast (gynecomastia);
hyperfunction of anterior lobus hypophisis (adenoma) leads to increase skeleton
(acromegaly).
2. Working hypertrophy develops in tissues consisting of stable undivided cells due to
increase of size it one. It may be often in cardiac muscle at some cardiac diseases, such
as: systemic hypertension, aortic valve disease (stenosis and insufficiency), mitral
insufficiency; hypertrophy of smooth muscle: cardiac achalasia (in esophagus), pyloric
stenosis (in stomach), and intestinal stricture; hypertrophy of urine bladder in
adenoma of prostatic glands.
3. Compensatory reparative hypertrophy: regeneration of the liver following partial
hepatectomy, regeneration of epidermis after skin abrasion; hypertrophy of
myocardium in postinfarctional cardiosclerosis.
4. Vicarious (substitutional) hypertrophy: following nephrectomy on one side in a
young patient there is compensatory hypertrophy as well as hyperplasia of the
nephrons of the other kidney.
5. Hypertrophic vegetations develop due to chronic inflammation in mucous
membranes (polyps and condilomas); lymphostasis leads to ingrowth of connective
tissue, examples of false hypertrophy. In wound healing, there is formation of
granulation tissue.
According to stage of adaptation two types of myocardial hypertrophy have been described:
Concentric. In concentric hypertrophy (clinically, no insufficiency) the musculature is
clearly enlarged, measuring till 1.8 cm, but chambers of the heart are not dilated.
Eccentric. In eccentric hypertrophy myocardium is enlarged but chambers of the heart are
dilated. This leads to hemodynamic disorder with cardiac insufficiency. It is called
myogenic dilatation.
The affected organ is enlarged and firm. For example: a hypertrophied heart of a patient with
systemic hypertension may weight 700-800 g as compared to average normal adult weight of 350 g.
There is enlargement of muscle fibers as well as of nuclei. At ultrastructural level, there is increased
synthesis.
Metaplasia
24
Metaplasia is defined as a reversible change of one type to another type of adult epithelial or
mesenchymal cells, usually in response to abnormal stimuli, and often reverts back to normal on
removal of stimulus. Metaplasia is broadly divided into 2 types:
A. Epithelial metaplasia. This is the more common type. The metaplastic changes may be patchy or
diffuse and usually result in replacement by stronger but less well-specialized epithelium. Some
common types of epithelial metaplasia following:
Squamous metaplasia: in bronchus in chronic smokers, in uterine endocervix in
prolapse of the uterus and in old age, in gall bladder in chronic cholecystitis with
cholelithiasis, in prostate in chronic prostatitis and estrogen therapy, in renal pelvis and
urinary bladder in chronic infection and stones; in vitamin A deficiency, apart from
xerophthalmia, there is squamous metaplasia in the nose, bronchi, urinary tract, lacrimal
and salivary glands.
Columnar metaplasia in which there is transformation to columnar epithelium:
intestinal metaplasia in healed chronic gastric ulcer, conversion of pseudostratified
columnar epithelium in chronic bronchitis and bronchiectasis to columnar type, in cervical
erosion.
B. Mezenhymal metaplasia. Less often, there is transformation of one adult type of mesenchymal
tissue to another.
Osseous metaplasia. Osseous metaplasia is formation of bone in fibrous tissue, cartilage
or myxoid tissue: in arterial wall in old age, in soft tissues in myositis ossificans, in cartilage
of larynx and bronchi in elderly people, in scar of chronic inflammation of prolonged
duration, in the fibrous stroma of tumor.
Cartilaginous metaplasia. In healing of fractures, cartilaginous metaplasia may occur
where there is undue mobility.
Dysplasia
Dysplasia means “disordered cellular development”, often accompanied with metaplasia and
hyperplasia, it is therefore also referred to as atypical hyperplasia. Epithelial dysplasia is
characterized by cellular proliferation and cytological changes, which include:
Hyperplasia of epithelial layers.
Disorderly arrangement of cells from basal layer to the surface layer.
Cellular and nuclear pleomorphism.
Increased nucleocytoplasmic ratios.
Nuclear hyperchromatism.
Increased mitotic activity.
The two most common examples of dysplastic changes are the uterine cervix and
respiratory tract.
Healing
Healing is the body response to injury in an attempt to restore normal structure and function.
The process of healing involves 2 distinct processes:
Complete regeneration (restitution), denoting the replacement of injured cells by
cells of the same type, sometimes leaving no residual trace of the previous injury, and
Incomplete regeneration (substitution) or replacement by connective tissue, or
fibroplasia, which leaves a permanent scar. In most instances, both processes contribute to
repair. In addition, both regeneration and fibroplasia are determined by essentially similar
mechanisms involving cell migration, proliferation, and differentiations, as well as cellmatrix interactions.
Depending upon their capacity to divide, the cells of the body can be divided into 3 groups:
Labile cells. These cells continue to multiply throughout life under normal physiologic
conditions. These include: surface epithelial cells of epidermis, alimentary tract, respiratory
tract, urinary tract, vagina, cervix, uterine endometrium, hematopoietic cells of bone
marrow and cells of lymph nodes and spleen.
Stable cells. These cells decrease or lose their ability to proliferate after adolescence but
retain the capacity to multiply in response to stimuli throughout adult life. These include:
parenchymal cells of organs like liver, pancreas, kidneys, adrenal and thyroid;
mesenchymal cells like smooth muscle cells, fibroblasts, vascular endothelium, bone and
cartilage cells.
Permanent cells. These cells lose their ability to proliferate around the time of birth.
These include: neurons of nervous system, skeletal muscle and cardiac muscle cells.
25
Forms:
Cellular: bones, epidermis, mucous membrane, connective tissue, endothelium,
hemopoetic system, and limfoid tissue.
Intracellular: myocardium, skeletal muscles, ganglious cells and central nervous system
(CNS).
Mixed: liver, kidneys, lungs, pancreas, endocrine organs, smooth muscles, vegetative
nervous system (VNS).
Types:
1. Physiological regeneration is the process of replacement that occurs due to physiologic necrosis
(erythrocytes, mucosa).
2. Reparative regeneration (complete, incomplete with regenerative hypertrophy) is the regeneration
after some pathologic necrosis.
3. Pathologic regeneration is the slow (hyporegeneration) or pathologically absence one,
hyperregeneration or metaplasia (change in cell type).
Repair
Repair is the replacement of injured tissue by fibrous tissue. Two processes are involved in
repair:
1. Granulation tissue formation.
2. Contraction of wounds.
Repair response takes place by participation of mesenchymal cells (consisting of connective
tissue stem cells, fibrocytes and histiocytes), endothelial cells, macrophages, platelets, and the
parenchymal cells of the injured organ.
Granulation tissue formation
The following 3 phases are observed in the formation of granulation tissue.
1. Phase of inflammation. There is acute inflammatory response with exudation of plasma,
neutrophils and some monocytes within 24 hours.
2. Phase of clearance. Combination of proteolytic enzymes liberated from neutrophils, autolytic
enzymes from dead tissues cells, and phagocytic activity of macrophages clear of the necrotic tissue,
debris and red blood cells.
3. Phase of ingrowth of granulation tissue. This phase consists of 2 main processes: angiogenesis or
neovascularisation and formation of fibrous tissue.
Angiogenesis (neovascularisation). Formation of new blood vessels at the site of injury
takes place by proliferation of endothelial cells from the margins of severed blood vessels. The process
of angiogenesis takes place under the influence of the following:
1. Endothelial cell growth factors.
2. Some components of matrix like type IV collagen.
Fibrous tissue formation. The new fibroblasts originate from fibrocytes as well as by
mitotic division of fibroblasts. Some of these fibroblasts have morphologic and functional
characteristics of smooth muscle cells (myofibroblasts). Collagen fibrils begin to appear by about 6th
day. As maturation proceeds, more and more of collagen is formed while the number of active
fibroblasts and new blood vessels decreases. This results in formation of inactive looking scar known
as cicatrisation.
Contraction of wounds. The wound starts contracting after 2-3 days and the process is
completed by the 14th day. In order to explain the mechanism of wound contraction, a number of
factors have been proposed:
1. Dehydration as a result of removal of fluid.
2. Contraction of collagen.
3. Discovery of myofibroblasts.
Wound healing
Healing of skin wounds provides a classical example of combination of regeneration and
repair described above.
Two types of factors influence the wound healing: those acting locally and those acting in
general.
Local factors: infection, poor blood supply to wound, foreign bodies including sutures
interfere with healing and cause intense inflammatory reaction and infection; exposure to
ionizing radiation; exposure to ultraviolet light; type, size and location of injury.
26
Systemic factors: age, nutrition, systemic infection, uncontrolled diabetes, hematological
abnormalities.
This can be accomplished in one of the following two ways:
1. Healing by first intention (primary union).
2. Healing by second intention (secondary union).
Healing by first intention (primary union)
This is defined as healing of a wound, which has the following characteristics:
Clean and uninfected.
Surgically incised.
Without much loss of cells and tissue.
Edges of wound are approximated by surgical sutures.
The sequence of events in primary union is described below:
1. Initial hemorrhage. Immediately after injury, the space between the approximated
surfaces of incised wound is filled with blood, which then clots and seals the wound
against dehydration and infection.
2. Acute inflammatory response. This occurs within 24 hours with appearance of
polymorphs.
3. Epithelial changes. The basal cells of epidermis from both the cut margins start
proliferating and migrating towards incisional space in the form of epithelial spurs.
4. Organization. By 3rd day, fibroblasts also invade the wound area. By 5th day, new
collagen fibrils start forming. In 4 weeks, the scar tissue with scanty cellular and
vascular elements, a few inflammatory cells and epithelialised surface is formed.
5. Suture tracks. Each suture track is a separate wound and incites the same
phenomena as in healing of the primary wound.
Healing by second intention (secondary union)
This is defined as healing of a wound having the following characteristics:
Open with a large tissue defect, at times infected.
Having extensive loss of cells and tissues.
The wound is not approximated by surgical sutures but is left open.
The sequences of events in secondary union are as under:
Initial hemorrhage.
Inflammatory phase. There is an initial acute inflammatory response followed by
appearance of macrophages, which clear off the debris as in primary union.
Epithelial changes. As in primary healing, the epidermal cells from both the margins of
wound proliferate and migrate into the wound in the form of epithelial spurs.
Granulation tissue. The main bulk of secondary healing is by granulations. Granulation
tissue is formed by proliferation of fibroblasts and neovascularisation from the adjoining
viable elements.
Wound contraction. Contraction of wound is an important feature of secondary healing, not
seen in primary healing.
Presence of infection. Bacterial contamination of an open wound delays the process of
healing due to release of bacterial toxins that provoke necrosis, suppuration and
thrombosis.
Complications of Wound Healing
Infection.
Implantation (epidermal) cyst.
Pigmentation.
Deficient scar formation.
Incisional hernia.
Hypertrophied scars and keloid formation.
Excessive contraction.
Neoplasia.
Hematological abnormalities.
27
HEMODYNAMIC DISTURBANCES
These are considered 2 broad headings
Disturbances in the volume of the circulating blood. These include hyperemia and
congestion, hemorrhage and shock
Circulatory disturbances of obstructive nature. These are thrombosis, embolism,
ischemia and infarction.
Hyperemia and congestion
Hyperemia and congestion are the terms used for increased volume of blood within dilated
vessels of an organ or tissue the increased volume from arterial and arteriolar dilatation being
referred to as hyperemia or active hyperemia, whereas the impaired venous drainage is called
venous congestion or passive hyperemia. The capillaries and veins are dilated paralytically
and filled with blood.
Arterial or active hyperemia is caused by an increased supply of blood from arterial
system. The affected tissue or organ is pink or red in appearance (erythema).
I. Common arterial or active hyperemia is a result
Of increasing volume of circulating blood (pletora).
Of increasing of amount of erythrocytes.
Vacatic (lat. – vacuum) because of decreased atmospheric pressure.
II. Local arterial hyperemia can be
Angioneurotic – because of dilatation of arteries and arterioles.
Collateral.
Hyperemia after anemia.
Vacatic.
Inflammatory.
In arterio-venous fistula.
Venous, or passive hyperemia, or congestion is caused by impediment to the exit of
blood through venous pathway. The dilatation of veins and capillaries due to impaired venous
drainage results in passive hyperemia or venous congestion, commonly referred to as congestion.
Congestion may be acute or chronic, the latter being more common and called chronic venous
congestion.
I. Common congestion or Systemic (General) venous congestion is engorgement of systemic veins.
It can be a result of
left-sided and right-sided heart failure
diseases of the lungs which interfere with pulmonary blood flow, like pulmonary fibrosis,
emphysema, etc.
cardiac decompensation.
II. Local congestion can be a result of
venous obstruction because of its thrombosis,
compression of venous vessel with tumor or ingrowth of connective tissue,
development of collateral blood circulation.
Morphology of congestion
Because of the increase in venous blood, organs become swollen and purplish. With long
continued over-distension, the wall of the venules shows reactive thickening and there is mild
intestinal fibrosis of the organs, giving them a very firm consistency. These changes are seen typically
in the kidney and spleen. Important additional changes are found in the lungs and liver.
Lungs. The lungs are burcly, congested and brownish in color. Pulmonary venous
engorgement leads to alveolar hemorrhage. Hemoglobin from intra-alveolar blood is transformed into
hemosiderin, which is then phagocytized by macrophages. These macrophages are known as heart
failure cells. Phagocytes full of brown pigment migrate into intestinal tissue and to the lymph nodus.
The sectioned surface is dark brown. It process in lungs is named as “brown induration” of the
lungs.
Spleen. Chronic venous congestion of the spleen occurs in right heart failure and in portal
hypertension from cirrhosis of liver. The spleen in early stage is moderately enlarged while in longstanding cases there is progressive enlargement and may weigh up to 500 g to 1000 g. The organ is
deeply congested, tense and cyanotic (“cyanotic induration of the spleen”). Sectioned surface is
gray tan. The red pulp shows congestion and marked sinusoidal dilatation with areas of recent and old
hemorrhages. These hemorrhages may get organized. This advanced stage seen more commonly in
28
hepatic cirrhosis is called congestive splenomegaly and is the commonest cause of
hypersplenism.
Liver. Chronic venous congestion of the liver occurs in right heart failure and sometimes due
to occlusion of inferior vena cava and hepatic vein. The liver is enlarged and tender and the capsule is
tense. Cut surface shows characteristic “nutmeg liver” due to red and yellow mottled appearance.
The changes of congestion are more marked in the centrolobular zone due to severe hypoxia than in
the peripheral zone. The centrolobular hepatocytes undergo degenerative changes, and eventually
centrolobular hemorrhagic necrosis may be seen. The peripheral zone of the lobule is less
severely affected by chronic hypoxia and shows some fatty change in the hepatocytes. If the patient
has periods of remission, the remaining liver cells may undergo compensatory hyperplasia. This
results in small, irregular, pale nodules alternating with areas of fibrosis – so-called cardiac cirrhosis.
It’s not true cirrhosis and does not causes hepatic failure.
Outcomes of congestion:
Edema.
Stasis.
Hemorrhage.
Thrombosis.
Induration of organs.
Atrophy of organs.
Hemorrhage
Hemorrhage (i.e. bleeding) is a discharge of blood from the vascular compairtment to the
exterior of the body or into nonvascular body spaces.
Mechanisms of hemorrhages
1. By destruction of the blood vessel’s wall (f.e. trauma, rupture of aneurysm).
2. By diapedesis of erythrocytes because of the increased permeability of the vascular wall (f.e.
intoxication, hypoxia).
3. By ulceration of the vessel’s wall (f.e. ulcer of stomach, necrosis of tumor, pulmonary tuberculosis).
Thus a severe decrease in the number of platelets (thrombocytopenia) or a deficiency of a
coagulation factor (e.g., factor VIII in hemophylia) is assosiated with spontaneous hemorrhages
unrelated to any apparent trauma.
Types of hemorrhages according to the site of origin
1. Cardiac, as following a penetrating heart wound.
2. Arterial, due to trauma and rupture of a dissecting aneurysm.
3. Capillary, which is usually due to trauma, inherent vessel wall weakness, or a coagulation defect.
4. Venous, which is usually caused by trauma or surgical operation, from esophageal varices.
Types of internal hemorrhages
Petechia – a small mucosal or serosal hemorrhage or minute punctate hemorrhage usually
in the skin or conjunctiva.
Purpura or hemorrhagic infiltration - the accumulation of some erythrocytes in tissue
between cells.
Ecchymoses or bruise - the superficial large extravasations of blood into the skin and
mucous membranes. Following a bruise in association with coagulation defect, an initially
purple discoloration of the skin turns green and then yellow before resolving, a sequence
that reflects the progressive oxidation of bilirubin released from the hemoglobin of
degraded of red blood cells. A good example of an eccxymosis is a “black eye”.
Hematoma - a grossly visible localized accumulation of the blood in the soft tissue.
Types of hemorrhages in body cavities
Hemothorax – hemorrhage in the pleural cavity.
Hemopericardium – hemorrhage in the pericardium cavity.
Hemoperitoneum – hemorrhage in the abdomen cavity.
Hemoarthrosis – hemorrhage in the joint cavity.
External hemorrhages may be such as:
29
Melena is deposition of the blood in the faces (excrement or stool) due to hemorrhage from
ulcer of stomach, polip or ulcer of intestines.
Hemoptyesis is hemorrhage from lungs.
Metrorrhagia is hemorrhage from uterus.
Outcomes of hemorrhages
Coagulation of the blood.
Organization and incapsulation of the hematoma.
Brown cystic formatiom (in cerebral hematoma due to accumulation of hemosiderin).
Purulent fusion of the hematoma.
In cases of death from acute massive hemorrhage, the most significant postmorten changes are
gross rather then microscopic and consists in generalized pallor of tissue, collapse of the great veins,
and a flabby, shrunken, gray spleen.
A sudden loss of 33% of blood volume may cause death, while loss of upto 50% of blood
volume over a period of 24 hours may not be necessarily fatal. However chronic blood loss generally
produces an iron deficiency anemia, whereas acute hemorrhage may lead to serious immediate
consequences such as hypovolemic shock.
Ischemia
Ischemia is a loss of blood supply, which occurs when arterial flow is impeded by
atherosclerosis or by thrombi, or by some other causes. Ischemia is the most common cause of
hypoxia.
Types of ischemia
Angiospastic (reflex).
Obstructive.
Compressive.
Because of redistribution of blood.
Morphologic features
The primary response of acute ischemia is cellular swelling or edema with dilation of the
endoplasmic reticulum, dissociation of polysomes into monosomes, swelling of
mitochondria, and also increased concentration of water, sodium, and chloride and
decreased concentration of potassium into the cytoplasm. If the duration of ischemia is
short, the structure and the function of tissue may be restored.
If ischemia persists, irreversible injury ensures with severe vacuolization of the
mitochondria including their christae, extensive damage to cytoplasm membranes, and
swelling of lysosomes. When the lesion is continuous, infarction, atrophy or sclerosis may
develop.
Infarction
Infarction is an area of ischemic necrosis within a tissue or an organ, produced by occlusion of
either its arterial supply or its venous drainage.
Types of infarctions:
Ischemic (white) infarction is encountered with arterial occlusion and in solid tissues
(spleen).
Red (hemorrhagic) infarction is encountered with venous occlusion, in tissue as with
double circulation, and in tissue previously congested (lung, intestinum).
White infarction with hemorrhagic halo (kidneys, heart).
According to their age, infarcts are classified as:
Recent or fresh.
Old or healed.
According to the propagation it may be
Total (when the whole organ is affected).
Subtotal (when only a part of the organ is affected).
Microinfarct (when observed only microscopically).
Pathogenesis
The process of infarction takes place as follows:
Localised hyperemia due to local anoxemia.
30
Within a few hours, the affected part becomes swollen due to edema and hemorrhage.
Cellular changes such as cloudy swelling and degeneration appear early.
There is progressive autolysis of the necrotic tissue and hemolysis of the red cells.
An acute inflammatory reaction and hyperemia appear at the same time in the surrounding
tissues.
Blood pigments, hematoidin and hemosiderin, liberated by hemolysis is deposited in the
infarct.
Following this, there is progressive ingrowth of granulation tissue from the margin of the
infarct.
Morphologic manifestations
Myocardial infarction usually develops due to thrombosis of coronary artery. This
infarction shows coagulative necrosis of myocardial cells. Almost no blood is seen in the vessels. The
nuclei of muscle’s fibers and stroma cells are absent. The peripheral portion of the infarction has been
invaded by acute inflammatory cells, which act as scavengers and remove the dead cells.
It is white infarction with hemorrhagic halo. It is classically irregular shape with hemorrhagic
infiltration.
Infarction of the lungs. Embolism of the pulmonary arteries may produce pulmonary
infarction, though not always. The pulmonary infarcts are classically wedge-shaped with base on the
pleura, hemorrhagic, variable in size, and most often in the lower lobes. Fibrinous pleuritis usually
covers the area of infarct. Cut surface is dark purple and may show the blocked vessel near the apex of
the infarcted area. Old organized and healed pulmonary infarcts appear as retracted fibrous scars. The
characteristic feature is coagulative hemorrhagic necrosis of the alveolar walls.
Renal infarction is common, found in upto 5% of autopsies. Renal infarcts are often
multiple and may be bilateral. Characteristically, they are pale or anemic and wedge-shaped with base
resting under the capsule and apex pointing towards the medulla. Generally, a narrow rim of
preserved renal tissue under the capsule is spared because it draws its blood supply from the capsular
vessels. The affected area shows characteristic coagulative necrosis of renal parenchyma i.e. there are
shadows of renal tubules and glomeruli without intact nuclei and cytoplasmic content.
Infarction of the spleen is one of the common sites for infarction. Splenic infarction results
from occlusion of the splenic artery or its branches. Splenic infarcts are often multiple. They are
characteristically pale or anemic and wedge-shaped with their base at the periphery and apex pointing
towards hilum. Coagulative necrosis and inflammatory reaction are seen.
Occlusion of an artery or vein may have little or no effect on the involved tissue or it may cause
death of the tissue and, indeed, of the individual. The major determinates include:
The nature of the vascular supply.
The rate of development of the occlusion.
The vulnerability of the tissue to hypoxia.
Clinical significance of infarction
Most of the cardiovascular deaths result from myocardial and cerebral infarction. Pulmonary
infarction is an extremely common complication in a variety of clinical settings. Ischemic necrosis
(gangrene) of the lower extremities is a relatively unusual clinical problem in the population at large
but is a major concern in diabetes melitus.
Stasis
Stasis (stasis - stop) is arrest of blood flow in the vessels of microcirculatory system
(capillaries). The capillaries and veins are dilated paralytically and filled with blood. In the lumen of
some capillaries the homogenous eosinophilic masses can be seen. They are columns of erythrocytes
sticked together, which is called prestasis. Sludge syndrome (phenomenon) is regarded as a type of
stasis. It is characterized by sticking of erythrocytes, leukocytes and thrombocytes to each other,
which is accompanied by blood viscosity increase.
Stasis may be discirculatory as a result of venous hyperemia or ischemia. Causes of stasis:
Physical factors (temperature elevation, cold).
Chemical factors.
Infection.
Infectious-allergic factors.
Autoimmune factors.
Short stasis is reversible, long one causes hyaline thrombi formation, vascular permeability
increase, edema, bleeding.
31
Isolated vein spasm may cause leukostasis, accumulation of erythrocytes within venules (small
veins) and capillaries. It is observed in hypoxia. In shock, leukostasis may be generalized, but as a rule
it is localized in the venules.
Microcirculation disturbances. There are four links in microcirculation:
1. The link of inflow and distribution of the blood (arterioles and precapillaries).
2. Intermediate (exchange) link (capillaries).
3. Depot link (postcapillaries and venules).
4. Drainage link (lymphatic capillaries and postcapillaries). The function of
microcirculation is exchange between the blood and tissue. Pathology of
microcirculatory system is formed of vascular, intravascular and extravascular
changes.
Vascular changes are those in the thickness and shape of the vessels, angiopathies with
disturbance of vascular permeability as a result of hypoxia.
Intravascular changes manifest as different disturbances of blood rheology (sludge, prestasis,
stasis). They are observed in shock of different origin.
Extravascular changes are perivascular edema, hemorrhage, lymphostasis on the lymph
vessels.
Thrombosis
Thrombosis is a pathologic process, which denotes the formation of a clotted mass of blood
within the noninterruptured vascular system.
Influences predisposing to thrombosis:
1. Injury to endothelium;
2. Alterations in the normal blood flow;
3. Alterations in the blood coagulation system (hypercoagulability).
Mechanisms of formation
Agglutination of platelets. Platelets adhere to the endothelium and to each other forming a
projecting mass;
Agglutination of erythrocytes. If the rate of the blood flow is slow, as in veins, red cells are
entangled so that the lumen is occluded;
Coagulation of fibrinogen. In front and behind the platelet mass the blood stagnates.
Further formation of fibrin takes place resulting in a large solid coagulum. The thrombus
extends in either direction to the nearest junction;
Precipitation of plasma proteins. With a slow blood flow in the joining vessel more fibrin is
formed by the platelets at the tip of the thrombus, thus occluding the joint vessel. Blood
stagnates in the joining vessel and thrombosis forwards to the next joining vessel. There
may be a succession of thrombotic episodes – a propagating thrombus.
Types of thrombi
According to the degree of the lumen obstruction, thrombi may be:
Occlusive thrombi most commonly develop in small arteries and veins.
Wall-attached or parietal thrombi develop in large arteries and heart cavities.
Axial.
Globe-shaped (in the heart).
According to the morphology
Thrombi may be of various shapes, size and composition depending upon the site of origin and
it is attached to the vascular wall; it is dense, with corrugated surface. It is composed of branching
bars of stuck thrombocytes and bands of fibrin with erythrocytes and leukocytes located between
them.
Morphological types of thrombi
White thrombus – consists mainly of platelets, fibrin and leukocytes; forms slowly in
rapid circulation of the blood (usually in the arteries);
Red thrombus – consists of platelets, fibrin and excessive amount of erythrocytes; forms
rapidly at slow blood circulation (usually in veins). Venous thrombi are dark-red colored
dry masses with dim surface.
Mixed or laminated thrombus – has laminated structure, contains white and red
elements of thrombus (usually forms in veins, aneurysms of aorta and heart). Mixed
32
thrombus consists of core or head (white thrombus), body (white and red) and tail (has
construction of red thrombus). Core is connected with endothelium. Mixed thrombus is of
gray-red color with rough dim surface, fixed to the intima of the vessel. Body and tail are
located freely in the vessel’s lumen.
Hyaline thrombus consists of precipitating plasma proteins, destructed erythrocytes,
leukocytes and thrombocytes. They do not contain fibrin. They resemble hyaline and are
located in the microcirculatory bed.
Agonal thrombus – consists of the yellowish fibrin and localizes in the apex of the right
ventricle of the heart and may extend into pulmonary artery. It is formed in the last minutes
of the life when the death occurs slowly. Red clot forms in case of the rapid death.
The distinguishing features between thrombi formed in rapidly-flowing arterial circulation and
slow-moving venous blood are given in Table 3.
TABLE 3. Distinguishing Features of Arterial and Venous Thrombi.
FEATURE
ARTERIAL THROMBI
VENOUS THROMBI
1. Blood flow
Formed in rapidly-flowing blood Formed in slow-moving blood in of arteries and heart
veins
2. Sites
Common in coronary, cerebral, Common in superficial varicose
iliac and femoral arteries
veins, deep leg veins, popliteal,
femoral and iliac veins
3.
Formed following endothelial cell Formed following venous stasis,
Thrombogenesis injury, e.g. in atherosclerosis
e.g. in abdominal operations, childbirth
4. Development
5. Macroscopy
6. Microscopy
7. Effects
Usually mural, not occluding the Usually occlusive, take the cast of
lumen completely, may propagate. the vessel in which formed, may
propagate in both directions.
Grey-white, friable with lines of Red-blue with fibrin strands and
Zahn on surface.
lines of Zahn.
Distinct lines of Zahn composed Lines of Zahn with more abundant
of platelets, fibrin with entangled red cells.
red and white blood cells.
Ischemia leading to infarcts, e.g. Thromboembolism, edema, skin
of heart, brain etc.
ulcers, poor wound healing.
Red thrombi (ante-mortem) have to be distinguished from postmortem clots (Table 4).
TABLE 4. Antemortem Thrombi versus Postmortem Clots
ANTEMORTEM THROMBI
1. Dry, granular, firm and friable
2. Adherent to the vessel wall
POSTMORTEM CLOTS
Gelatinous, soft and rubbery
Weakly attached to the vessel wall
3. May or may not fit their vascular contours Take the shape of vessel or its bifurcation
4. The surface contains apparent lines of
Zahn.
The surface is “chicken fa” yellow covering
the underlying red “currant jell”.
33
Clinical effects of thrombosis
These depend upon the site, rapidity of formation, and nature of thrombi.
1. Cardiac thrombi. Large thrombi in the heart may cause sudden death by mechanical obstruction of
blood flow or through thromboembolism to vital organs.
2. Arterial thrombi. These cause ischemic necrosis of the deprived part (infarct), which may lead to
gangrene. Sudden death may occur following thrombosis of coronary artery.
3. Venous thrombi (Phlebothrombosis). These may cause various effects:
Thromboembolism.
Oedema of area drained.
Poor wound healing.
Skin ulcer.
Painful thrombosed veins.
Painful white leg.
Thrombophlebitis migrans in cancer.
4. Capillary thrombi. Microthrombi in microcirculation may give rise to disseminated intravascular
coagulation (DIC).
Outcomes of the thrombosis
A.Favourable outcomes:
Aseptic autolysis (dissolution) by fibrinolytic system, proteinolytic enzymes of macrophages
and leukocytes.
Organization by the replacement of connective tissue.
Recanalization is the re-establishment of the vascular lumen through occluding thrombus.
Incorporation or vascularization means restoration of the circulation in the vessel because
of the formation of the new vessels through the thrombotic mass.
Petrification or dystrophy calcification – accumulation of the calcium salts in the
thrombotic masses.
B.Unfavourable outcomes:
Thromboembolism.
Septic autolysis.
Propagation with following obstruction of some critical vessel.
Embolism
Embolism is the passage through the venous or arterial circulations of any material capable
of lodging in a blood vessel and they’re by obstructing the lumen. The transported intravascular mass
detached from its site of origin is called an embolus.
Types of embolism
According to localization:
Small blood circulation.
Large blood circulation.
System of vena portae.
According to the direction of the movement of embolus:
Orthograde (by blood flow)
Retrograde (against blood flow). Metastasis of the carcinoma prostate in the spine takes
place.
Paradoxical (emboli arising in the venous circulation may by pass the lungs by travelling
through an incompletely closed foramen ovale, subsequently blocking flow in systemic
arteries).
According to the material of the embolus:
1. Solid:
Thromboembolism.
Atheroembolism.
Tissue (cellular) embolism due to necrosis of tumor, damaged tissue.
Bacterial embolism.
Embolism by parasites.
Embolism by foreign bodies.
2. Liquid:
Fat embolism.
34
Amniotic fluid embolism.
3. Gaseous:
Decompression sickness (caisson disease).
Air embolism.
Thromboembolism
A detached thrombus or part of thrombus constitutes the most common type of embolism.
These may arise in the arterial or venous circulation:
The effects of arterial emboli depend upon their size, site of lodgement, and adequacy of
collateral circulation:
Infarction.
Gangrene.
Arteritis and mycotic aneurysm.
Myocardial infarction.
Sudden death.
The most significant effect of venous embolism is obstruction of pulmonary arterial
circulation leading to pulmonary embolism.
Pulmonary thromboembolism
Pulmonary embolism is the most common and fatal form of venous thromboembolism in
which there is occlusion of pulmonary arterial tree by thromboemboli. Pulmonary emboli are more
common in hospitalised or bedridden patients. The majority of emboli arise from the deep veins of the
low extremities; most of the fatal ones arise from the ileofemoral veins. Condition that favor the
development of pulmonary thromboembolism are:
Stasis (heart failure, chronic venous insufficiency).
Injury (trauma, surgery, parturition).
Hormonal imbalance (oral contraceptive use).
Advanced age.
Immobilization (orthopedic, paralysis, bed rest).
Sickle cell disease.
Pathogenesis
Detachment of thrombi from any of the above-mentioned sites produces a thromboembolus
that flows through venous drainage into the large veins draining into right side of the heart.
If the thrombus is large, it is impacted at the bifurcation of the main pulmonary artery
(saddle embolus), or may be found in the right ventricle or its outflow tract.
More commonly, there are multiple emboli, or a large embolus may be fragmented into
many smaller emboli.
Paradoxical embolism may occur by passage of an embolus from right heart into the
left heart through atrial or ventricular septal defect.
Consequences of thromboembolism
1. Consequences of pulmonary embolism. These include:
Pulmonary Syndrome (Infarction). The pulmonary syndrome clinically resembles
pneumonia. Pleural effusion is common and often bloody. Pathologically, pyramidal segments of
hemorrhagic infarction are seen at the periphery of the lung. Obstruction of terminal branches
(endarteries) leads to central pulmonary hemorrhage.
Circulatory Syndrome (Without Infarction). Embolism produces pulmonary
hypertension by mechanical blockage of the arterial bed. Reflex vasoconstriction and bronchial
constriction due to release of vasoactive substances may contribute to a reduction in the size of the
functional pulmonary vascular bed. Whether a patient develops the pulmonary or the circulatory
syndrome depends on the thromboembolic load and the availability of circulatory reserve of the
bronchial arteries. Pulmonary hypertension may lead to chronic cor pulmonale and pulmonary
arteriosclerosis. Numerous small emboli may obstruct most of the pulmonary circulation resulting in
acute right heart failure (Acute cor pulmonale).
Massive Pulmonary Embolism. Massive pulmonary emboli typically cause sudden
obstruction of blood flow through one or both of the major pulmonary arteries. The patient often goes
into shock immediately - resumably because of certain: neurologic reflexes - and may die within
minutes. This catastrophe is characteristically precipitated when a patient who has been recuperating
from surgery gets out of bed for the first time.
35
2. Consequences of emboli in peripheral arteries. The heart is the most common
source of systemic emboli, which usually arise from mural thrombi (in atrial fibrilation, mitral valve
disease, myocardial infarction, left ventricular aneurysm, heart failure of any etiology,
cardiomyopathy) or diseased valves (bacterial endocarditis, marantic endocarditis).
Clinical and morphological features: arterial emboli to the brain cause strokes; in the
mesenteric circulation they cause infarction of the bowel; embolism of an artery of the legs leads to
sudden pain, absence of pulse, and a cold limb; renal artery embolism may infarct the entire kidney
but more commonly results in small peripheral infarcts; coronary artery embolism results in
myocardial infarctions.
Thus, the effects and sites of arterial emboli are in striking contrast to venous emboli, which
are often lodged in the lungs.
Atheroembolism. Atheromatous plaques, especially from aorta, may get eroded to form
atherosclerotic emboli. The pathologic changes and their effects are:
Ischemia, atrophy and necrosis,
Infarcts in the affected organs,
Gangrene in the lower limbs,
Hypertension.
Fat Embolism. Obstruction of arterioles and capillaries by fat globules constitutes fat
embolism. If the obstruction in the circulation is by fragments of adipose tissue, it is called fat-tissue
embolism. Important causes are: trauma, inflammation of bones and soft tissues, fatty liver,
pancreatitis, extrinsic fat or oils introduced into the body.
Consequence of fat embolism:
Pulmonary fat embolism. Frozen section is essential for confirmation of globules by fat
stains such as Sudan dyes (Sudan black, Sudan III and IV), oil red O and osmic acid.
Systemic fat embolism. Some of the fat globules may pass through the pulmonary
circulation such as via patent foramen ovale, arteriovenous shunts in the lungs and vertebral venous
plexuses, and get lodged in the capillaries of organs like the brain, kidney, skin.
Gas Embolism. Two main forms of gas embolism are air embolism and decompression
sickness.
Air Embolism occurs when air is introduced into venous or arterial circulation.
Causes of venous embolism include: operations on head and neck, and trauma, obstetrical
operations, intravenous infusion of blood and fluid, angiography. The effects of venous air
embolism depend upon the following factors: amount of air usually 100-150 ml of air entry
is considered fatal, rapidity, position of the patient during or soon after entry of air. The air
bubbles may ascend into the superior vena cava if the position of head is higher than the
trunk (e.g. in upright position) and reach the brain. General condition of the patient e.g. in
severely ill patients, as little as 40 ml of air may have serious results.
Causes of arterial embolism include: cardiothoracic surgery and trauma, paradoxical air
embolism, arteriography. The effects of arterial air embolism are certain characteristic
features: marble skin due to blockage of cutaneous vessels, air bubbles in the retinal vessels
seen ophthalmoscopically, pallor of the tongue due to occlusion of a branch of lingual
artery, coronary or cerebral arterial air embolism may cause sudden death by much smaller
amounts of air than in the venous air embolism.
Decompression Sickness. This is a specialized form of gas embolism known by various
names such as caisson's disease, divers' palsy or aeroembolism. Decompression sickness is
produced when the individual decompresses suddenly, either from high atmospheric pressure to
normal level, or from normal pressure to low atmospheric pressure.
Clinical effects of decompression sickness are of 2 types:
1. Acute form occurs due to acute obstruction of small blood vessels in the vicinity of joints and
skeletal muscles. The condition is clinically characterized by the following:
“The bends”, as the patient doubles up in bed due to acute pain in joints, ligaments and
tendons.
The “chokes” resulting in acute respiratory distress.
Cerebral effects may manifest as vertigo, coma, and sometimes death.
2. Chronic form is due to foci of ischemic necrosis throughout body, especially the skeletal system:
Vascular necrosis of bones.
Neurological symptoms.
Lung involvement.
Skin manifestations.
Other organs like parenchymal cells of the liver and pancreas may show lipidvacuoles.
36
Diagnosis: at autopsy, the right heart is punctured without taking it out. The cavity of the
cardiac sac should be preliminary filled with water. Air discharge and foamy blood are observed.
Amniotic Fluid Embolism. This is the most serious, unpredictable and unpreventible cause
of maternal mortality. During labour and in the immediate post-partum period, the contents of
amniotic fluid may enter the uterine veins and reach right side of the heart resulting in fatal
complications. Notable changes are seen in the lungs such as hemorrhages, congestion, edema and
changes of ARDS, and dilatation of right side of the heart. These changes are associated with
identifiable amniotic fluid contents within the pulmonary microcirculation.
The cause of death may be a result of the following mechanisms:
Mechanical blockage of the pulmonary circulation,
Anaphylactoid reaction to amniotic fluid,
Disseminated intravascular coagulation (DIC),
Hemorrhagic manifestations due to thrombocytopenia and afibrinogenemia.
Shock
Shock is defined as a clinical state of cardiovascular collapse characterized by (1) an acute
reduction of effective circulating blood volume and (2) an inadequate perfusion of cells and tissues
The final result is hypotension and cellular hypoxia and, if uncompensated, may lead to
impaired cellular metabolism and death.
Primary or initial shok. It is transient and usually a benign vasovagal attack resulting from
sudden reduction of venous return to the heart caused by peripheral pooling of blood. It can occur
immediately following trauma, severe pain or emotional over-reaction such as due to fear, sorrow or
surprise. The attack usually lasts for a few seconds or minutes
Secondary of true shok. This is the form of shock, which occurs due to hemodynamic
derangements with hypoperfusion of the cells. This type of shock is the true shock, which is commonly
referred to as “shock” if not specified, and is the type described below.
According to etiology and pathogenesis shock is classified as:
1. Hypovolemic. Reduction in blood volume induces hypovolemic shock. The causes of hypovolemia
include: a) Severe hemorrhage (external or internal) e. g. in trauma, surgery, b) Fluid loss e. g. in severe
burns, crush injury to a limb, persistent vomitings and severe diarrhea causing dehydration.
2. Cardiogenic. Acute circulatory failure with sudden fall in cardiac output from acute diseases of the
heart without actual reduction of blood volume (normovolemia) results in cardiogenic shock. The
causes include:
Deficient emptying (myocardial infarction, rupture of the heart, cardiac arrhythmias).
Deficient filling (cardiac tamponade from hemopericardium).
Obstruction to the outflow (pulmonary embolism, ball valve thrombus).
3. Septic. Severe bacterial infections or septicemia induce septic shock:
Gram-negative septicemia (endotoxic shock,) e.g. infection with E. coli, Proteus Klebsiella,
Pseudomonas and bacteroides. Endotoxins of gram-negative bacilli have been implicated
as the most important mediator of septic shock
Gram-positive septicemia (exotoxic shock) is less common e.g. infection with streptococci,
pneumococci caused by endotoxins).
4. Anaphylactic (immediate reaction of hypersensitivity).
5. Neurogenic (in intoxication with hypnotic preparations, ganglioblockers, narcotics).
6. Shock developing in hormonal insufficiency (thyrotoxic shock, myxedema, adrenal insufficiency).
Pathogenesis
Stages of Shock. Deterioration of the circulation in shock is a progressive phenomenon and
can be divided arbitrarily into 3 stages:
1. Non-progressive (initial compensated reversible) shock. In the early stage of shock, an attempt is made to
maintain adequate cerebral and coronary blood supply by redistribution of blood. This is achieved by
activation of various neurohormonal mechanisms causing widespread vasoconstriction and by fluid
conservation by the kidney.
2. Progressive decompensated shock. This is a stage when the patient suffers from some other stress or
risk factors besides persistence of the shock so that there is progressive deterioration.
3. Decompensated (irreversible) shock. When the shock is so severe that in spite of compensatory
mechanisms and despite therapy and control of etiologic agent, which caused the shock, no recovery
takes place it is called decompensated or irreversible shock.
Shock morphology
37
Three main pathological processes are observed in shock:
DIC (disseminated intravascular coagulation) syndrome.
Hemorrhagic diathesis.
Liquid cadaver blood.
Microscopically, it is characterized by generalized spasms of the vessels, microthrombosis,
signs of increased vascular permeability in microcirculatory system, hemorrhages, degenerations,
necroses connected with hypoxia and damaging effect of endotoxins.
Morphologic features of complications in Shock
Shock kidney: degeneration and necrosis in proximal canals with development of necrotic
nephrosis (or symmetrical cortical necroses are possible), which results in acute renal
insufficiency.
Shock liver: glycogen amount in the hepatocytes decreases, hydropic degeneration and
centrolobular necroses resulting in acute hepatic insufficiency develop. Combination of
renal and hepatic insufficiency is called hepatorenal syndrome.
Shock lung: atelectasis foci, serous-hemorrhagic edema, stases and thromboses in the
microcirculatory bed resulting in acute respiratory insufficiency.
Shock heart: degeneration and necrosis in cardiomyocytes, reduction in glycogen amount,
fat degeneration, and necrotic foci.
Shock gastrointestinal: the hypoperfusion of alimentary tract may result in mucosal and
mural infarction called hemorrhagic gastroenteropathy.
Shock brain: Hypoxic changes in the brain. Ischemic neurons appear shrunken and have
eosinophilic cytoplasm. The pericellular spaces are dilated because of edema. Rarification of
brain tissue presents.
Similar changes occur in nervous, endocrine systems, and immune organs.
Shock morphology depends not only on the cause of the shock but also on its stage. At the early
stage, disturbances of hemodynamic and DIC syndrome are noted. At the last stages degenerative and
necrotic process occurs.
Intensive transfusion therapy of shock masks clinicomorphological picture. But the constant
features are liquid cadaver blood irrespective of the composition of transfused fluids. Blood clots in
the cardiac cavities and vessels are characteristic for terminal states of nonshock origin. So blood
composition is a criterion for differential diagnosis.
Clinical Features
The classical features of decompensated shock are characterized by depression of 4 vital
processes:
Very low blood pressure.
Subnormal temperature.
Feeble and irregular pulse.
Shallow and sighing respiration.
Renal dysfunction in shock is clinically characterized by a phase of oliguria due to ATN and a
later phase of diuresis due to regeneration of tubular epithelium. With progression of the condition
the patient may develop stupor, coma and death.
Disseminated intravascular coagulation
Disseminated intravascular coagulation (DIC) is pathological syndrome, which is
characterized by formation of disseminated blood clots in the microcirculatory bed (often in
combination with simultaneous reduction of blood coagulability) causing hemorrhages. It often
develops in complicated pregnancy, profuse uterine bleedings, large injuries, anemia,
thrombocytopenia, leukemia, in 36 - 50% of cases of asphyxia in premature children.
Stages of DIC
At the first stage it is characterized by generalized increase of blood coagulation in the
microvessels. Large number of fibrin clots is formed. They close the vessel
(fibrinoembolism).
At the second stage the amount of thrombocytes, fibrinogen, prothrombin in the blood
decreases sharply because they have already been used at the first stage with the resultant
consumption coagulopathy. Thus, hemorrhagic syndrome develops.
38
At the third stage fibrinolysis activation takes place in response to generalized increase
of coagulation occurring at the first stage, which makes hemorrhagic syndrome more
severe.
In severe cases the three stages develop simultaneously. Disturbance in blood clotting is
accompanied by stasis, opening of arteriovenous shunts, capillary paralysis, decrease in arterial
pressure. Degenerative and necrotic changes develop in parenchymatous organs.
Morphological changes in DIC syndrome
Large amount of fibrin thrombi and emboli in the small vessels of the liver, red pulp of
spleen, adrenals, brain, lungs, kidneys, placenta, thymus.
Mucoid swelling, fibrinoid swelling and fibrinoid necrosis with endothelium desquamation
in the walls of small arteries.
In thrombosis of microcirculatory bed, vital processes of blood-tissue metabolism stop.
Under these conditions organ pathology is not distinct, general changes (like toxicosis or
shock) develop.
In thrombosis of larger arteries, organ pathology prevails, i.e. acute renal or hepatic
insufficiency, shock lung, brain edema, and myocardial infarction.
DIC results in hemorrhages in different organs; those in the capsule are most frequent.
Edema
Edema may be defined as abnormal and excessive accumulation of fluid in the interstitial
tissue spaces and serous cavities.
Edema fluid lies free in the interstitial space between the cells and can be displaced from one
place to another. Edema fluid may be:
Transudate, which is more often the case such as in edema of cardiac and renal disease.
Exudate such as in inflammatory edema.
The differences between transudate and exudate are tabulated in Table 5.
TABLE 5 Differences between Transudate and Exudate
FEATURE
TRANSUDATE
EXUDATE
Definition
Infiltrate of blood plasma without Edema of inflamed tissue associated
changes in endothelial permeability with increased vascular permeability
Character
Non inflammatory edema
Inflammatory edema
Protein content Low (less than 3 g/dl): mainly High (more than 3 g/ dl), readily
albumin, low fibrinogen, has no coagulates due to high content of
tendency to coagulate
fibrinogen and other coagulation
factors
Cells
Few cells, mainly mesothelial cells Many cells (inflammatory as well as
and cellular debris
parenchymal)
Examples
Edema in congestive cardiac failure Purulent exudates such as pus
Types of edema
1. According to the presence or absence of inflammation
Inflammatory.
Noniflammatory is a result of (1) increase in intravascular hydrostatic pressure, (2) fall of
colloid osmotic pressure of the plasma, (3) impairment in the flow of lymph, and (4) renal
retention of salt and water.
2. According to propagation
39
Generalized (anasarca).
Localized (hydrothorax or pleural effusion, hydropericardium, hydroperitoneum or ascitis).
Localization of edema
Subcutaneous edema of the lower parts of the body is a manifastation of cardiac failure.
Renal edema as a result of renal dysfunction or nephrotic syndrome tends to be generalized,
affecting all parts of the body.
Pulmonary edema is usually confined to the lower lobes.
Edema of the brain (cerebral edema) may be localized to the region of the focal lesions of
generalized involving the entire brain, as in encephalitis, hypertensive crises, and the
obstruction to the venous outflow of the brain.
Increase in interstitial fluid amount. If transudate accumulates in subcutaneous fat it is
called anasarca, in the heart cavity - hydropericardium, in the pleural cavity - hydrothorax,
in the abdominal cavity- ascites, in the testis - hydrocele.
Edema develops in the patients with cardiovascular, kidneys, liver, allergic diseases, infections,
pathologic conditions of pregnancy (hestosis), in vein thrombosis, lymph congestion, disturbances of
nervous trophism, etc.
In these diseases, the following changes are observed: 1) those in hydrostatic blood pressure,
2) those in colloid osmotic pressure of blood plasma, 3) vascular wall permeability increases, 4)
retention of water and electrolytes.
These factors accompany each other in the majority of cases, but as a rule one of them prevails,
e.g. mechanical or congestive edema develops as a result of increase in hydrostatic pressure in
microvessels and in fluid filtration.
Oncotic edema results from reduction in colloid-osmotic pressure in the blood plasma.
Membranogenic edema is associated with the increase in capillary permeability, which results in
plasma protein exit and its accumulation in the tissues. Electrolyte edema results from retention of
water and electrolytes. Lymphogenic edema is caused by lymph congestion.
The outcome of edema is favorable, the fluid resolves, but prolonged edema can result in
degeneration, atrophy, sclerosis.
Reduction in interstitial fluid amount (exicosis) may occur in rapid loss of great amount
of fluid (cholera, prolonged diarrhea).
INFLAMMATION
Inflammation is fundamentally a protective response whose ultimate goal is to rid the
organism of both the initial cause of cell injury and the consequences of such injury, the necrotic cells
and tissues. Inflammation of an organ is usually named by adding the suffix-it is to its Latin name.
The agents causing inflammation may be following:
Physical agents (heat, cold, radiation, mechanical injury).
Chemical agents (organic and inorganic poisons).
Infective agents (bacteria, viruses, parasites).
Immunological agents (cell-mediated and antigen-antibody reactions).
Classic clinical signs of inflammation:
Heat (calor).
Redness (rubor).
Edema (tumor).
Pain (dolor).
Loss of Function (functio laesa).
Types of Inflammation
1. Morphological types:
Alterative.
Exssudative.
Proliferative (productive).
2. According to the Type of Tissue Reaction:
Normergic.
Hypoergic.
Hyperergic.
3. According to Etiology:
Specific.
Non-specific.
40
4. According to the Duration:
Acute.
Subacute.
Chronic.
5. Types of Exssudative Inflammation:
Pseudomembranous.
Serous.
Fibrinous (croupous and diphtheric).
Suppurative (abscess, phlegmon, empyema).
Putrificative.
Hemorrhagic;
Catarrhal (serous, mucous, and suppurative).
Mixed.
6. Types of Proliferative Inflammation:
Interstitial.
With formation of polyps and condylomas.
Around parasites.
Granulomatous.
7. Specific Inflammation:
A. Accompanied Diseases:
Tuberculosis.
Syphilis.
Leprosy.
Scleroma.
Glanders.
B. Features:
Definite pathogenic organism.
Changing of tissue reactions.
Chronic wavy course.
Prevailed proliferative inflammation with formation of granulomas.
Necrosis of exsudate (primary and secondary).
Acute inflammation
Acute inflammation is the immediate and early response to an injurious agent.
The major components of acute inflammation:
Alterations in vascular caliber that lead to an increase in blood flow.
Structural changes in the microvasculature that permits plasma proteins and leukocytes to
leave the circulation.
Emigration of the leukocytes from the microcirculation and their accumulation in the focus
of injury.
Vascular changes in acute inflammation:
Vasoconstriction mediated by both neurogenic and chemical mediator system.
Vasodilation caused by the release of specific mediators is responsible for the redness and
warmth at sites of tissue injury.
Stasis with leukocytic orientation along the vascular endothelium (leukocytic margination).
Increased vascular permeability, leading to the escape of a protein-reach fluid into the
interstitium with following edema.
Extravasation of leukocytes from the vascular lumen into the interstitial tissue as a result of
the following steps: (1) in the lumen: margination, rolling, and adhesion; (2) transmigration
across the endothelium (diapedesis), and (3) migration in the interstitial tissues toward a
chemotactic stimulus.
Phagocytosis is the process of engulfment and internalization of foreign agents or injuried
cell material and cells that possess this function are reffered to as phagocytic cells.
Phagocytosis and the release of enzymes by neutophils and macrophages constitute two of the
major benefits derived from the accumulation of the leukocytes at the inflammatory focus.
Phagocytosis involves three distinct but inter-related steps: (1) recognition and attachment of the
particle to be ingested by the macrophage; (2) its engulfment, with subsequent formation of a
phagocytic vacuole; (3) killing or degradation of the ingested material.
Systemic effects of acute inflammation.
41
The account of acute inflammation given above is based on local tissue responses. However,
acute inflammation is associated with systemic effects as well as under:
Fever occurs due to bacteriemia.
Leucocytosis commonly accompanies the acute inflammatory reactions, usually of the range
of 15,000-20,000/nl. Usually, in bacterial infections there is neutrophilia; in viral
infections lymphocytosis; and in parasitic infestations, eosinophilia. Typhoid fever, an
example of acute inflammation, however, induces leucopenia with relative lymphocytosis.
Lymphangitis-lymphadenitis is one of the important manifestations of localized
inflammatory injury.
Shock may occur in severe cases. Systemic activation of coagulation pathway may occur
leading to microthrombi throughout the body and result in DIG, bleeding and death.
Morphologic patterns in acute inflammation
1. Serous inflammation.
It is marked by the outpouring of a thin fluid that is derived from either the bloodstream or
the secretions of mesothelial cells.
The skin blister resulting from a bum or viral infections represents a large accumulation of
serous fluid, either within or immediately beneath the epidermis of the skin.
Serous exudates contain until 2% protein and less quantity cells (neutrophils, macrophages,
desquamative epithelium).
Serous inflammation locates in serous membranes (polyserositis at rheumatic diseases,
autointoxications - uremia), in mucus (serous rinitis), in skin (streptococcus infections,
herpes, burn), seldom in internal organs (serous pneumonia).
Outcomes are favorable, because exudates resolves.
2. Catarrhal inflammation. A surface inflammation associated with greatly increased secretion of
clear mucus. Catarrhal inflammation may be serous, supurative and mixed.
3. Hemorrhagic inflammation. Where the damage is severe, actual rupture of all blood vessels
occurs, with hemorrhage the most striking feature (acute hemorrhagic pneumonia occasionally
occurring in fatal cases of influenza, plague, anthrax).
4. Fibrinous inflammation or Pseudomembranous inflammation.
It is inflammatory response of mucous surface (oral, respiratory, bowel) to toxins of
diphtheria or irritant gases. As a result of denudation of epithelium, plasma exudes on the
surface where it coagulates, and together with necrosed epithelium, forms false membrane
that gives this type of inflammation its name.
Causes: streptococcus, pneumococcus, immune complex, shigella, corynebacterium
diphtheriae.
It is localized in mucus and cerous membranes by forming fibrinoid films, in lungs at
croupouse pneumonia.
Fibrinous exudate contains large amount of fibrin, neutrophils, and macrophages.
According to the type of epithelium on which inflammatory process develops and depth of
necrosis there are two types of fibrinous inflammation: croupous and diphtheric
fibrinous inflammation.
Usually croupous inflammation develops on the columnar epithelium. In this case the
fibrinous membranes unfix easily, without any effort.
Diphtheric fibrinous inflammation develops on the squamous or intermediate epithelium,
when the fibrinous membranes unfix with difficulties.
Histologically fibrin appears as an eosinophilic network of threads or sometimes as an
amorphous coagulum.
This exudate may be remove by fibrinolysis, and other debris by macrophages.
This process, called resolution may restore normal tissue structure.
Conversion of the fibrinous exudate to scar tissue is called organization.
A fibrinous exudate is characteristic of inflammation in body cavities, such as the
pericardium and pleura.
Conversion of the fibrinous exudate to scar tissue (organization) within the pericardial sac
will lead either to opaque fibrous thickening of the pericardium and epicardium in the area
of exudation or, more often, to the development of fibrous strands that bridge the
pericardial space (obliteration).
5. Suppurative or purulent inflammation.
Causes: stafiloccocus, gonococcus, streptococcus, meningococcus, pseudomonas
aeruginosa.
42
It is characterized by the production of large amounts of pus or purulent exudate consisting
of a lot of neutrophils, necrotic cells, and edema fluid.
There are the following types of purulent inflammation:
Abscess – localized inflammation.
When acute bacterial infection is accompanied by intense neutrophilic infiltrate in the
inflamed tissue, it results in tissue necrosis.
A cavity is formed which is called an abscess and contains purulent exudate or pus and the
process of abscess formation is known as suppuration.
Pus is creamy or opaque in appearance and is composed of numerous dead as well as living
neutrophils, some red cells, fragments of tissue debris and fibrin. In old pus, macrophages
and cholesterol crystals are also present.
Furuncle is an acute inflammation via hair follicles in the dermal tissues.
Carbuncle is seen in untreated diabetics and occurs as a located abscess in the dermis and
soft tissues of the neck.
Acute abscess has a central region appeared as a mass of necrotic white cells and tissue
cells, a zone of preserved neutrophils around this necrotic area, and area characterized with
vascular dilation, parenchymal and fibroblastic proliferation, indicating the beginning of
repair. The internal wall of abscess is called pyogenic membrane.
Chronic abscess has internal pyogenic membrane, medium – granulation tissue, external –
fibrous tissue membrane.
An abscess may be discharged to the surface due to increased pressure inside or may
require drainage by the surgeon. Fistula can be formed.
Due to tissue destruction, resolution does not occur but instead healing by fibrous scarring
takes place.
Phlegmon – a diffuse purulent inflammation.
It may be densed and soft.
Phlegmon frequently occurs along the muscular fibres, tendons, fascias, vascular-nerves
fibres and in subcutaneous fat.
Cellulitis is a diffuse inflammation of soft tissues resulting from spreading effects of
substances like hyaluronidase released by some bacteria.
Empyema – a purulent inflammation of serous membranes (empyema of pleura, empyema of
gall bladder and urinary bladder and so on).
6.Putrificative is assosiated with anaerobic infection and characterized by numerous
necrosis.
7. Ulcer. Ulcer is a local defect on the surface of an organ produced by inflammation. In the
acute stage, there is infiltration by polymorphs with vasodilatation while long-standing ulcers develop
infiltration by lymphocytes, plasma cells and macrophages with associated fibroblastic proliferation
and scarring.
Fate of acute inflammation
The acute inflammatory process can culminate in the following:
Complete resolution. This means complete return to normal tissue following acute
inflammation. It occurs when tissue changes are slight and the cellular changes are
reversible, e.g. resolution in lobar pneumonia.
Healing by connective tissue replacement (fibrosis). This takes place when the tissue
destruction in acute inflammation is extensive so that there is tissue regeneration but
actually there is healing by fibrosis.
Progression to suppuration. Pyemia is the dissemination of small septic thrombi in the
blood, which cause their effects at the site where they are lodged. This can result in pyemic
abscesses or septic infarcts. Pyemic abscesses are multiple small abscesses in various organs
such as in cerebral cortex, myocardium, lungs, and renal cortex, resulting from very small
emboli fragmented from septic thrombus.
Progression of the tissue response to chronic inflammation. Acute inflammation may
progress to chronic one in which the processes of inflammation and healing proceed side by
side.
Chronic inflammation
Chronic inflammation is considered to be inflammation of prolonged duration, in which
active inflammation, tissue destruction, and attempts at healing are proceeding simultaneously.
Chronic inflammmation arises under the following settings:
43
Chronic inflammation following acute inflammation - when the tissue destruction is
extensive, or the bacteria survive and persist in small numbers at the site of acute
inflammation, e.g. in osteomyelitis, pneumonia terminating in lung abscess.
Recurrent attacks of acute inflammation - when repeated bouts of acute inflammation
culminate in chronicity of the process, e.g. in recurrent urinary tract infection leading to
chronic pyelonephritis, repeated acute infection of gall bladder leading to chronic
cholecystitis.
Chronic inflammation starting de novo - when the infection with organisms of low
pathogenicity is chronic from the beginning, e.g. infection with Mycobacterittm
tuberculosis.
Under certain conditions, immune reactions are set up against the individual’s own tissues
leading to autoimmune diseases.
General features of chronic inflammation
1. Mononuclear cell infiltration, which include macrophages, lymphocytes and plasma cells,
eosinophils and mast cells.
2. Tissue destruction or necrosis is brought about by activated macrophages by release of a variety of
biologically active substances.
3. Proliferative changes. As a result of necrosis, proliferation of small blood vessels and fibroblasts is
stimulated resulting in formation of inflammatory granulation tissue. There are four components of
this process:
formation of new blood vessels (angiogenesis),
migration and proliferation of fibroblasts,
deposition of extracellular matrix,
maturation and organization of the fibrous tissue, also known as remodeling.
Types of chronic inflammation:
I. Nonspecific, when the irritant substance produces a non-specific chronic inflammatory reaction
with formation of granulation tissue and healing by fibrosis, e.g. chronic osteomyelitis, chronic ulcer.
II. Specific, when the injurious agent causes a characteristic histologic tissue response, e.g.
tuberculosis, leprosy, syphilis, scleroma.
However, for a more descriptive classification, histological features are used for classifying
chronic inflammation into 3 corresponding types:
1. Chronic nonspecific inflammation.
It is characterized by nonspecific inflammatory cell infiltration, e.g. chronic osteomyelitis,
chronic lung abscess.
A variant of this type of chronic inflammatory response is chronic suppurative
inflammation in which infiltration by polymorphs and abscess formation is additional
features, e.g. actinomycosis.
The inflammatory cell infiltration consists of lymphocytes, monocytes, plasmocytes,
eosinophils and other cells.
2. Chronic nonspecific interstitial inflammation with formation of polyps and pointed condyloma.
It occurs on the mucous membranes and in the areas borderline with squamous epithelium.
Polyps are the end-result of prolonged chronic irritation. Nasal, cervical, colorectal polyps
are common. Macroscopically they are gelatinous masses with smooth and shining surface.
Microscopically they are composed of loose edematous connective tissue containing some
mucous glands and varying number of inflammatory cells (lymphocytes, plasmocytes,
eosinophils).
Condyloma acuminatum is commonly located on the coronal sulcus on the penis or the
perineal area. Condyloma is the growth of squamous cell epithelium and connective tissue
of the skin with appearance of numerous small papillas on the surface. In stroma there are
hyperemic vessels, infiltrates of lymphocytes and plasma cells with admixture of leukocytes.
3. Chronic granulomatous inflammation.
It is characterized by formation of granulomas, e.g. tuberculosis, leprosy, syphilis,
actinomycosis, sarcoidosis etc.
Granulomatous inflammation is the distinctive pattern of chronic inflammatory
reaction in which the predominant cell type is an activated macrophage with a modified
epithelial-like (epithelioid) appearance.
Granuloma is defined as a circumscribed, tiny lesion, about 1 mm in diameter, composed
predominantly of collection of modified macrophages called epithelioid cells, and rimmed
at the periphery by lymphoid cells.
44
The word “granuloma” is composed of granule meaning circumscribed granule-like
lesion, and -oma, which is a suffix commonly, used for true tumors but here indicates
inflammatory mass or collection of macrophages.
Epithelioid cells, so called because of their epithelial cell-like appearance, are modified
macrophages which are somewhat elongated, having pale-staining abundant cytoplasm,
lightly-staining slipper-shaped nucleus and the cell membrane of adjacent epithelioid cells
is closely apposed.
Besides the presence of epithelioid cells and lymphoid cells, granulomas may have giant
cells, necrosis and fibrosis:
The giant cells are formed by fusion of adjacent epithelioid cells and may have 20 or
more nuclei. These nuclei may be arranged at the periphery like horseshoe or ring or
clustered at the two poles (Langhans’ giant cells), or they may be present centrally
(foreign body giant cells).
Necrosis may be a feature of some granulomatous conditions, e.g. central caseation
necrosis of tuberculosis.
Fibrosis is due to proliferation of fibroblasts at the periphery of granuloma. The following
two factors favour the formation of granulomas:
1. Presence of poorly digestible irritant, which may be organisms like Mycobacterium
tuberculosis, particles of talc etc.
2. Presence of cell-mediated immunity to the irritant, implying thereby the role of
hypersensitivity in granulomatous inflammation.
Types of granulomas:
1. Infectious, and noninfectous.
2. Foreign body granulomas and immune granulomas.
Granulomatous inflammation is typical of reaction to poorly digestible agents elicited by
tuberculosis, leprosy, fungal infections, schistosomiasis, foreign particles, etc.
Microscopical examination of the spesific granulomas
In tuberculosis, the granuloma is reffered to as a tubercle and is classically characterized by
the presence of central caseous necrosis surraunded by epitelioid cells, limphocytes, plasma cells and
giant Langhance’s cells. In contrast, caseous necrosis is rare in other granulomatous diseases.
The syphilis granuloma is called gumma. Gumma consists of a central area of fibrinoid
or caseous necrosis surrounded by mononuclear inflammatory cells, mostly plasma cells,
lymphocytes, epitelioid cells, and seldom-giant Langhance’s cells. Around gumma forms the
granulations tissue and endovasculitis.
In tuberculoid leprosy, the epidermis contains confluent granulomas composed of
macrophages, plasma cells, and leprous Virhov’s cells. Leprous Virhov’s cells (or leprosy cells)
refer as large foamy macrophages within fatty vacuoles containing leprous mycobacteriums.
In rhinoscleroma of nose, the granuloma (scleroma) consists of the plasma cells, epithelioid
cells, lymphocytes, and hyaline sphere. Large macrophages with light cytoplasm containing Klebsiella
rhinoscleromatis (Mikulicz’s cells), sclerosis and hyalinosis take place.
Microscopical examination of the non- spesific granulomas
Proliferative inflammation around echinococcus of the liver. The area of the liver’s
tissue with destructive pink color shined chitinous membrane and surrounded necrotic tissue are
seen. In peripheral areas crowded lymphocytes, plasma cells, fibroblasts and single “giant cells of the
foreign bodies” can be found. In the outside –fibrous capsule.
Sarcoidosis. The tissue contains granulomas composed of epithelioid macrofages and only a
few lymphocytes and giant cells. There is no central necrosis.
The outcomes of chronic inflammation depend on the type of inflammation, morphofunctional
characteristic of the definite organ or tissue, where inflammation develops. Frequently sclerosis and
hyalinosis may develop.
IMMUNOPATHOLOGY
Immunopathological processes are pathological states, which are associated with
disturbances of structure and function of lymphoid tissue. Before studying the morphology
of immunogenesis disturbance it is necessary to know the normal immune morphology.
Central organs of immune system producing immune-competent cells are bone marrow
and thymus. The bone marrow contains progenitor cells for the other lymphoid organs. The
progenitor cells produced in the bone marrow circulate to the thymus or peripheral organs
45
of immune system, where they develop into more mature lymphoid cells. Populations of
bone marrow cells that may have recirculated back to the bone marrow can respond to
antigens and are called B-lymphocytes.
The thymus produces and differentiates small lymphocytes (T-lymphocytes).
Main peripheral organs of immune system are spleen; lymphatic nodes and gastrointestinal
associated lymphoid tissue (GALT) and bronchus associated lymphoid tissue (BALT). The
main immunocompetent cells are T-lymphocytes, B-lymphocytes, and macrophages.
There are T and B-zones in the peripheral organs of the immune system. Thus, in the
spleen, periarterial zone of the follicle is T-zone, marginal zone is inhibited by Blymphocytes. There are T-, B- lymphocytes and macrophages in the red pulp of the spleen;
In the lymphatic nodes, paracortical zone and peripheral zone of the follicle is T-zone,
cortical layer, light centers of the follicles age’s-zone. There are T-, B- lymphocytes and
macrophages in the medullar substance. Gastrointestinal associated lymphoid tissue
(GALT) and bronchus associated lymphoid tissue (BALT) have different immunocompetent
cells, i.e. T-lymphocytes, B-lymphocytes, macrophages without any zones.
The main T-cell function is to recognize “host” and “foreign” cells. Transmission of the
information is the main function of B-cells. Information transmission is carried out through
macrophage system. The function of B-cells is to produce antibodies. At this stage Blymphocytes are transformed into plasmoblasts and plasmocytes.
For identification of immunocompetent cells we use monoclonal antibodies to different
immune cells.
Cellular Components of the Immune Response:
T-cells are thymus-derived lymphocytes and produce (1) B cell growth factor; (2) B cell
differentiation factor; (3) colony stimulating factor; (4) fibroblast activating factor; (5)
Gamma-interferon; (6) interleukin-2; (7) interleukin-3; (8) leikocyte inhibition factor; (9)
lymphotoxin; (10) migration inhibition factor.
B-cells are defined as lymphocytes that bear membrane immunoglobulin and under
appropriate conditions differentiate into antibody-secreting cells.
Natural Killer Cells have capacity to recognize directly and kill various tumors and virusinfected in vitro.
Mononuclear Phagocyte is a general term applied to populations of phagocytic cells found
in virtually all organs and connective tissues. Among these cells are macrophages,
monocytes, Kupffer’s cells of the liver, and the so-called histiocytes. Macrophages are
dominant participants in subacute and chronic inflammatory reactions.
Human Major Histocompatability Complex or human leukocyte antigens are the main
target antigens during rejection of transplanted organs.
Pathology of Thymus
Thymus is the organ regulating the whole immune system. At immunogenesis disturbances we
usually see the following processes and pathology.
1. Accidental thymus transformation (involution), that is reduction in the size and mass due to
thymocyte migration to the peripheral immune organs and blood as well as due to their partial"
decomposition and absorption by macrophages (this is called apoptoses).
According to T. Ivanovskaya (1976), accidental involution consists of 5 stages.
Stage 1 – “holey clearing” - accumulation of lymphocytes around the macrophages. It occurs
in the cortex.
Stage 2 - transition of the lymphocytes from the cortex to the medullar substance. The
boundary between the layers is either poorly seen or not seen at all.
Stage 3 – “layer inversion”, when the cortex layer looks light, and medullar layer looks dark as
a result of transition of lymphocytes from the cortex to the medullar substance.
Stage 4 - Reduction in the lymphocyte amount in the both layers, reticular stroma growth.
Stage 5 - collapse of the lobe of the thymus and sclerosis and lobe atrophy.
Accidental transformation more often occurs in the newborn suffering from stress factors. The
more powerful is the stimulus, the more pronounced is the degree of involution. Accidental involution
occurs in infections, intoxications, in the children born from sick mothers. The process is reversible.
Elimination of pathological agent results in thymus normalization.
2. Thymus hyperplasia (thymolymphatic state, thymomegaly). The weight and the size of thymus are
considerably increased. Microscopic examination reveals a large number of immature lobules (zones
are not distinct). The density of the thymocytes is high. If this condition is accompanied by
46
hypoplasia of adrenal and sexual glands as well as narrow aorta and arteries, this pathological
process is called “thymolymphatic state”.
Sudden death syndrome (crib death) may occur in thymomegaly, it results from insufficiency
of T-lymphocytes of the cortex and medullar substance of the adrenal glands.
3. Thymus hypoplasia is characterized by absence of lobule division into cortical and medullar
substance, poor development, of reticuloepithelial component, responsible for hormonal function, as
well as lymphocyte component. As a rule thymus hypoplasia is typical for congenital immune
deficiency.
Changes of lymphoid tissue at antigen stimulation
In the thymus, different stages of accidental transformation are observed.
In the bone marrow the first hyperplasia of B- lymphocytes are observed, when it becomes
empty, as a result of increased transition of lymphocytes.
The reaction in peripheral lymphoid organs is similar. First, T-zones and B-zone
hyperplasia occurs. Macrophages and plasmatic cells appear as well as their blasts
producing immunoglobulins. Vascular endothelium is swollen; there are lymphocytes in the
lumen. After that both T and B-zones become empty. T-zone is characterized by “holey”
appearance. In B-zone density of the cells decreases. The lymphocytes either die or circulate
in the blood.
Reticuloepithelium hyperplasia and lympho-plasmocyte infiltration occur in the interstitial
tissue of the kidneys, pancreas, intestines, liver, and muscles.
Hypersensitivity reaction
A state of balance in the immune responses (humoral or cell-mediated) is essential for
protection against endogenous and exogenous antigens. Hypersensitivity is defined as a state of
exaggerated immune response to an antigen. The lesions of hypersensitivity (immunologic tissue
injury) are produced due to interaction between antigen and product of the immune response.
Immunologically Mediated Tissue Injury. An immune response that results in tissue
injury is broadly reffered to as “hypersensitivity” reaction and is associated with a group of diseases
categorized as immune and immunologically mediated disorders.
1. Type I Hypersensitivity (Immediate Type or Anaphylaxis) is manifested by a localized or
generalized reaction that occurs immediately (within minutes) after exposure to an antigen to which
the individual has previously become sensitized and is characterized by a specific cytotropic antibody
that binds to receptors on basophils and mast cells and reacts with specific antigen. This results in the
activation of mast cells and basophils and the release of performed (granule) products as well as the
synthesis of mediators.
2. Type II Hypersensitivity (Cytotoxic Type) reactions are caused by an antigen-antibody reaction
but, as the name implies, the antibodies formed are often cytotoxic and are directed against antigens
on cell surfaces or in connective tissues. Complement is required for many of cytotoxic events. Lysis
is mediated directly by complement or indirectly by opsonization or the chemotactic attraction of
phagocytic cells. Complement-independent reactions, such as antibody-dependent, cell-mediated
cytotoxisity also fall into this category.
3. Type III Hypersensitivity reactions involve tissue injury mediated by immune complexes. They
represent the classic example of immune complex-mediated injury in which antigen-antibody
complexes, which are usually not organ-specifiic, are formed in the circulation and mainly directly in
tissues. Once deposited in the tissues, these complexes induce an inflammatory response by
activating the complement system, consequently attracting neutrophils and macrophages. Activation
of these cells by the immune complexes,with the release of potent inflammatory mediators, is directly
responsible for the injury. Many human diseases, including anti-immune diseases such as systemic
lupus erythematosus, as well as most types of glomerulonephritis, appear to be mediated by type 111
hypersensitivity reactions.
4. Type IV Hypersensitivity or Cell-Mediated Immunity is defined as an antigen-elicited cellular
immune reaction that results in tissue damage and does not require the participation of antibodies. It
includes: (1) delayed type hypersensitivity; (2) T cell-mediated cytotoxisity, and (3) natural killer cellmediated cytotoxisity.
Depending upon the rapidity and duration the immune response, two distinct
forms of hypersensitivity reactions are recognized:
Hypersensitivity of Immediate reaction morphologically manifests itself by the picture
of acute immune inflammation, which develops rapidly, alteration and exudation stages prevail, and
proliferation increases slowly. The vessels and connective tissues are involved first. Alteration
manifests by mucoid, fibrinoid swelling and fibrinoid necrosis. The exudate is either fibrinous or
47
fibrino-hemorrhagic. Acute immune inflammations are observed in tuberculosis, syphilis. It is
responsible for vascular reaction in lupus erythematosus, glomerulonephritis, nodular periarteritis.
Hypersensitivity of Delayed reaction
Two types of cells take part in this reaction. They are sensibilized lymphocytes and
macrophages. Morphologically it manifests by chronic immune inflammation characterized by
lymphocyte-macrophage infiltration. When we see lymphocyte-macrophage infiltration accompanied
by vascular plasmorrhagic and degenerative processes we can conclude about immune inflammation.
The condition occurs in autoimmune diseases, tuberculosis, brucellosis, dermatitis, and
granulomatosis.
Reaction of transplant rejection resembles delayed hypersensitivity reaction. Transplant
antigens induce the production of antibodies and sensibilized lymphocytes, which infiltrate the
transplant. Microscopically, lymphohistiocyte infiltration is observed in the transplant. Cellular
infiltration causes the disturbance of blood circulation and edema; as a result degenerations and
necrosis of transplant develop. The neutrophils and macrophages appear in the transplant. Enzyme
destruction of the transplant begins which is followed by its rejection.
Immunodeficiency Diseases
Immunodeficiency disorders are classified into antibody (B cell), cellular (T cell), and
combined T and B cell deficiencies. In many cases functional defects are localized to particular points
in the ontogeny of the immune system. The defects are congenital or acquired and their precise
etiologies are often unclear.
1. Deficiencies of Antibody (B cell) immunity:
Congenital (Bruton’s) X-linked infantile hypogammaglobulinemia.
Transient hypogammaglobulinemia of infancy.
Common variable immunodeficiency.
Selective IgA deficiency.
2. Deficiencies of Cell-Mediated (T Cell) Immunity:
DiGeorge syndrome.
Chronic mucocutaneous candidosis.
3. Combined T and B Cell Deficiencies
4. Acquired Immunodeficiency.
Autoimmune diseases
Autoimmunity implies that an immune response has been generated against self-antigen
(autoantigens).
Central to the concept of autoimmunity is the breakdown in the ability of the immune
system to differentiate between self- and nonself-antigens.
An abnormal autoimmune response to self-antigens implies that there is a loss of immune
tolerance.
Tolerance is best looked on as a diversion of the immune system to an active state of
nonreactivity: that is, inhibitory products block the immune response.
The causes of autoimmune diseases are not clearly known.
Chronic viral infections, radiation and genetic factors may be responsible for them. Before
studying the pathogenesis of autoimmune diseases it is necessary to know the major
histocompatibility complex (MHC), which includes class 1, 2, 3 markers.
Class 2 MHC markers are also called HLA-Dr. There are a lot of autoimmune diseases,
which are connected, with genetic disturbances of HLA-Dr system. That is why HLA genes
are included into predisposing factors in the pathogenesis of autoimmune diseases.
In the pathogenesis of autoimmune diseases the following factors are
distinguished:
Predisposing (HLA genes, hormonal background, genetically dependent features of the
target cells).
Initiating (viral and bacterial infections, exposure of immune system and target organs to
chemical and physical factors).
Contributing (dysfunction of immune system, T-lymphocyte suppressor activity).
In the pathogenesis, 2 mechanisms can be distinguished; therefore all the autoimmune
diseases can be divided into 2 groups:
Group 1. Organospecific diseases. They are characterized by disturbance of physiological isolation of
the organs and tissues due to absence of immune tolerance. Lymphohistiocyte infiltration occurs in
the tissues (like at slow hypersensitivity reaction). The main organ specific diseases are:
48
Endocrine glands: Hashimoto’s (autoimmune) thyroiditis, Graves' disease, insulindependent diabetes mellitus, idiopathic Addison’s disease.
Alimentary tract: Autoimmune atrophic gastritis in pernicious anemia, ulcerative colitis,
Crohn’s disease.
Blood cells: Autoimmune hemolytic anemia, autoimmune thrombocytopenia,
Others Myasthenia gravis: Autoimmune orchitis, autoimmune encephalomyelitis,
Goodpasture’s syndrome, primary biliary cirrhosis, chronic active hepatitis, and
membranous glomerulonephritis.
Autoimmune skin diseases.
Group 2. Organ non-specific diseases. Primary disturbances in the immune system causing the loss of
ability to distinguish “own” and “foreign” antigens are:
Systemic lupus erythematous.
Rheumatoid arthritis.
Scleroderma (Progressive systemic sclerosis).
Polymyositis-Dermatomyositis.
Polyarteritis nodosa (PAN).
Sjogren’s syndrome.
Reiter’s syndrome.
Mixed connective tissue disease.
The diseases with autoimmune disturbances
In these diseases antigenic properties of the tissues change, which causes immune reaction
development.
Autoimmunization is responsible not for the beginning but the progress of the disease as
autoimmune antibodies appear during the disease.
It is observed in glomerulonephritis, hepatitis, chronic gastritis, burn disease,
rheumatism, hepatic cirrhosis.
Immune deficiency syndromes
Immune deficiency syndromes result from immune system insufficiency.
All immune deficiencies are divided into 2 groups: primary or congenital immune
deficiencies and secondary, acquired immune deficiencies.
Primary IDS may be understood as primary defects in development of the immune system.
Secondary ones results from diseases or drugs that affect immune system.
Primary IDS may be classified into following 4 general groups depending on the stage in
development at which the defect occurs:
- T-cell deficiencies.
- B-cell deficiencies.
- Combined (T-B-cell) deficiencies.
- Deficiency in inflammatory cells (agranulocytosis).
T-cell deficiencies manifests by agenesis, hypoplasia of the thymus and T-dependent
zones of the immune system. They are inherited according to autosome dominant type, e.g. MacCusic
syndrome. Except for the pathology of thymus and primary lymphatic tissue, defects of development
occur.
B-cell deficiencies. The type of inheritance is connected with X chromosome, e.g.
agammaglobulinemia – Bruton’s syndrome. The thymus is preserved. B-zones in the peripheral
lymphatic organs are absent. Immunoglobulins synthesis is absent.
Combined syndromes - insufficiency of cellular and humoral immunity (T- B-cell). This is
inherited according to autosome-recessive type, e.g. Gianzmann-Riniker syndrome
(agammaglobulinemia of Swiss type). Hypoplasia of thymus and peripheral lymphatic tissue.
Secondary deficiencies occur after full development of the immune system. Some of these
are secondary to immunosuppressive therapy, e.g. in tumors, autoimmune diseases,
glomerulonephritis, ect. Chronic virus infections and HIV (human immunodeficiency virus) may
cause secondary deficiencies. The aquired immunodeficiency syndrome (AIDS) has become
recognized as fatal and increasingly prevalent disease. AIDS exhibits a spectrum of clinical
manifestations, including an asymptomatic state with only laboratory evidence of immunodeficiency;
a prodromal state manifested by fever, weight loss, and lymphadenopathy, and the classic picture of
opportunistic infections and Kaposi’s sarcoma. The major laboratory features of AIDS are
lymphopenia and the loss of circulating T4 (helper/amplifier) lymphocytes. The etiologic agent of
49
AIDS is now known to be a retrovirus originally called HTLV-111 (human T cell leukemia/lymphoma
virus).
NEOPLASIA
General pathomorphology of neoplasia
The term “neoplasia” means new growth; the new growth produced is called “neoplasm”
or “tumor”.
However, all “new growth” is not neoplasms since examples of new growth of tissues and
cells also exist in the processes of embriogenesis, regeneration on repair, hyperplasia and
hormonal stimulation.
Neoplastic cells lose control and regulation of replication and form an abnormal mass of
tissue.
Satisfactory definition of neoplasm or tumor is “a mass of tissue formed as a result of
abnormal, excessive, uncoordinated, autonomous and purposeless proliferation of cells”.
The tumors are classified according to histogenetic principles with the account of their
morphological structure, localization, peculiarity of their structure in a definite organ,
benign or malignant character.
The classification was suggested as an international one by the Committee on Tumor
Nomenclature of the International Anticancer Union. According to this classification, there are 7
groups of tumors; their total number exceeds 200.
1. Epithelial tumors without specific localization (nonorganspecific).
2. Tumors of endocrine and exocrine, glands as well as epithelial integument (organspecific).
3. Mesenchymal tumors.
4. Tumors of melanin-forming tissue.
5. Tumors of nervous system and brain membranes.
6. Tumors of blood system.
7. Teratomas.
The suffix “-oma” is added to denote benign tumors. Malignant tumors of epithelial origin are
called carcinomas, while malignant mesenchymal tumors are named sarcomas (sarcos = fleshy).
Some examples contrary to this concept are: melanoma for carcinoma of the melanocytes, hepatoma
for carcinoma of the hepatocytes, lymphoma for malignant tumor of the lymphoid tissue, and
seminoma for malignant tumor of the testis.
Tumors composed of a single type of parenchymal cells that differentiate towards more than
one cell line are called mixed tumors. Teratomas, on the other hand, are made up of a number of
parenchymal cell types arising from totipotent cells derived from more than one germ cell layer.
Choristoma refers to the ectopic rests of normal tissue. Hamartoma is a mass of disorganised-but
mature cells of tissues indigenous to the particular site. The currently used classification of tumors is
based on the histogenesis (i.e. tissue of origin) and on the anticipated behavior.
Characteristics of tumors
The characteristics of tumors are described under:
I. Macroscopic features.
II. Microscopic features.
III. Growth rate.
IV. Local invasion (Direct spread).
V. Metastasis (Distant spread).
Based on these characteristics, contrasting features of benign and malignant tumors are
summarized in Table 6.
I. Macroscopic features
Almost all tumors have a different color, texture and consistency as compared to the
surrounding tissue of origin. Gross terms such as papillary, fungati, infiltrating,
hemorrhagic, ulcerative and cystic are used to describe the macroscopic appearance of the
tumors.
Benign tumors are generally spherical or ovoid in shape. They are encapsulated or wellcircumscribed, freely movable, more often firm and uniform, unless secondary changes like
hemorrhage or infarction supervene.
Malignant tumors are usually irregular in shape, poorly-circumscribed and extend into
the adjacent tissues. Secondary changes like hemorrhage, infarction and ulceration are seen
more often. Sarcomas typically have fish-flesh like consistency while carcinomas are
generally firm.
50
II. Microscopic features:
These are: microscopic pattern, cytomorphology of neoplastic cell (differentiation and
anaplasia), angiogenesis and tumor stroma, and inflammatory reaction.
1. Microscopic patten:
The tumor cells may be arranged in a variety of patterns in tumors, e.g.
The epithelial tumors generally consist of acini, sheets, columns or cords of epithelial
tumor cells that may be arranged in solid or papillary pattern.
The mesenchymal tumors have mesenchymal tumor cells lying separated from each
other usually by the intercellular substance such as cartilaginous matrix in chondroma,
osteoid in osteosarcoma, reticulin network in soft tissue sarcomas etc.
Hematopoetic tumors such as leukemias and lymphomas often have none or little
stromal support.
Generally, most benign tumors and low-grade malignant tumors reduplicate the normal
structure of origin more closely.
Other cellular deviations from the normal cellular arrangement in malignant tumors are:
loss of basal orientation (polarity), altered alignment of tumor cells to each other, and
stromal invasion by tumor cells.
2. Tumor Cytomorphology (Differentiation and Anaplasia)
Differentiation is defined as the extent of morphological and functional resemblance of
parenchymal tumor cells to corresponding normal cells. If the deviation of neoplastic cell in
structure and function is minimal as compared to normal cell, the tumor is described as
“well-differentiated” such as most benign and low-grade malignant tumors. “Poorly
differentiated”, “undifferentiated” or “dedifferentiated” are synonymous terms for poor
structural and functional resemblance to corresponding normal cell.
Anaplasia is lack of differentiation and is a characteristic feature of most malignant
tumors.
As a result of anaplasia, following noticeable morphological and functional alterations in the
neoplastic cells are observed:
1) Pleomorphism means variation in size and shape of the tumor cells. The extent of
cellular pleomorphism generally correlates with the degree of anaplasia.
2) Nucleocytoplasmic changes. These are as under:
Generally, the nuclei of malignant tumor cells are enlarged, so that the nucleocytoplasmic
ratio is increased.
The nuclei too, show variation in size (anisonucleosis) and shape in malignant tumor cells.
Characteristically, the nuclear chromatin of malignant cell is increased and coarsely
clumped, referred to as hyperchromatism. Besides, a prominent nucleolus or nucleoli may
be present in these nuclei reflecting increased nucleoprotein synthesis.
It is most important to identify abnormal and atypical mitotic figures such as tripolar,
quadripolar and multipolar spindles in malignant tumour cells because increased number
of normal mitoses may be present in non-neoplastic proliferations such as in hematopoietic
cells of the bone marrow, intestinal epithelium, hepatocytes etc.
Multinucleate tumor giant cells or giant cells containing a single large and bizarre nucleus,
possessing nuclear characters of the adjacent tumor cells, are another important feature of
anaplasia.
The cytoplasm of tumor cells in better-differentiated cancers and in benign tumors may
show the normal constituents from which the tumor is derived. But the more anaplastic
tumor cells lose such features.
3) Genetic abnormalities. All tumor cells have abnormal genetic composition and on
division they transmit the genetic abnormality to their progeny. Most malignant
tumors show aneuploidy.
4) Functional changes. Structural anaplasia in tumors is accompanied with functional
anaplasia. The functional abnormality in neoplasms may be quantitative, qualitative, or
both.
Generally, benign tumors and better-differentiated malignant tumors continue to function well
qualitatively, though there may be quantitative abnormality in the product, e.g. large or small amount
of collagen produced by benign tumors of fibrous tissue, keratin formation in well-differentiated
squamous cell carcinoma. In more anaplastic tumors, there is usually quantitative fall in the product
made by the tumor cells, e.g. absence of keratin in anaplastic squamous cell carcinoma.
51
Hormones or hormone-like substances may be produced by certain tumors quite unrelated to
the endocrine glands, called ectopic hormone production, e.g. oat cell carcinoma of the lung can
secrete ACTH and ADH.
3. Angiogenesis and Tumor Stroma.
The connective tissue along with its blood supply forms the supportive framework on which
the parenchymal tumor cells grow and receive nourishment. In order to provide nourishment to
growing tumor, new blood vessels are formed from pre-existing ones (angiogenesis) that is probably
stimulated by secretion of tumor angiogenesis factors from the parenchymal tumor cells such as
vascular endothelial growth factor (VEGF). However, if the tumor outgrows its blood supply as
occurs in rapidly growing tumors, its core undergoes ischemic necrosis. If the tumor is almost entirely
composed of parenchymal cells, it is called medullary, if there is excessive connective tissue stroma, it
is referred to as desmoplasia and the tumor is hard or scirrhous.
4. Inflammatory Reaction.
At times, prominent inflammatory reaction is present in and around the tumors. It could be
the result of ulceration in the cancer when there is secondary infection. However, some tumors show
chronic inflammatory reaction, chiefly of lymphocytes, plasma cells and macrophages, and in some
instances, granulomatous reaction, due to cell-mediated immunologic response by the host in an
attempt to destroy the tumor, e.g. seminoma testis, malignant melanoma of the skin,
lymphoepithelioma of the throat, medullary carcinoma of the breast, Warthin’s tumor of salivary
glands etc.
TABLE 6: Contrasting Features of Benign and Malignant Tumors.
FEATURES
BENIGN
(DIFFERENTIATED)
MALIGNANT (UNDIFFERENTIATED)
Poorly-circumscribed and irregular
2. Surrounding tissue
Encapsulated or wellcircumscribed
Often compressed
3. Size
4. Secondary changes
Usually small
Occur less often
Often larger
Occur more often
Often poor resemblance to tissue of origin
2. Basal polarity
Usually resembles the tissue of
origin closely
Retained
3. Pleomorphism
Usually not present
Often present
4. Nucleo-cyto-plasmic
ratio
5. Anisonucleosis
6. Hyperchromatism
Normal
Increased
Absent
Absent
Generally present
Often present
7. Mitoses
Mitotic figures increased and are
generally atypical and abnormal
Present with nuclear atypia
9. Cytoplasm
May be present but are always
typical mitoses
May be present but without
nuclear atypia
May show normal constituents
10. Function
Usually well maintained
MACROSCOPIC
FEATURES
1. Boundaries
II. MICROSCOPIC
FEATURES
1. Pattern
8. Tumor giant cells
Usually invaded
Often lost
Normal cytopiasmic elements are reduced
or lost
May be retained, lost or become abnormal
52
III. GROWTH RATE
Usually slow
Usually rapid
IV. LOCAL
INVASION
Often compresses the
surrounding tissues without
invading or infiltrating them
Absent
Usually infiltrates and invades the
adjacent tissues
V. METASTASIS
Frequently present
III. Growth rate
The tumor cells generally proliferate more rapidly than the normal cells. In general, benign
tumors grow slowly and malignant tumors rapidly. The rate at which the tumor enlarges depends
upon 3 main factors:
1. Rate of division and destruction of tumor cells. The rate of division of tumor cells depends upon 2
factors - proportion of cells undergoing mitosis (milotic index), and the duration taken to complete
the mitotic cell cycle.
2. Non-neoplastic elements within the tumors. These are the connective tissue stroma, abundant
mucoid material, cartilaginous matrix etc all of which add to the bulk of the tumors.
3. Degree of differentiation. In general, rate of growth of malignant tumor is directly proportionate to
the degree of differentiation. Rarely, a malignant tumor such as choriocarcinoma and malignant
melanoma may disappear spontaneously from the primary site, possibly due to necrosis caused by
good host immune attack, only to reappear as secondaries elsewhere in the body. The regulation of
tumor growth is under the control of growth factors secreted by the tumor cells.
Depending on the degree of the tumor differentiation, there are different types of its growth:
expansive, apposition, infiltrating (invasive).
At expansive growth the tumor grows from itself moving away the surrounding tissues.
This type of growth is slow, and is characteristic benign tumors.
Apposition growth is due to transformation of normal cells to tumor ones.
In infiltrating growth the cells of the tumor invade normal tissues and destroy them (so
called destructive growth).
In relation to the lumen of the hollow organ, the growth of the tumor may be endophytic or
exophytic.
Endophytic growth is infiltrating growth of the tumor deep into the wall of the organ.
Exophytic growth is expansive growth of the tumor to the cavity of the organ.
According to the number of foci of tumor development, they can be unicenter (one focus) and
multicenter (several foci).
IV. Local invasion (direct spread)
Most benign tumors form encapsulated or circumscribed masses that push aside the
surrounding normal tissues without actually invading, infiltrating or metastasising. Malignant tumors
also enlarge by expansion. But, they are distinguished from benign tumors by invasion, infiltration
and destruction of the surrounding tissue, besides distant metastasis. Often, cancers extend through
tissue spaces, permeate lymphatics, blood vessels, and perineural spaces and may penetrate a bone by
growing through nutrient foramina. More commonly, the tumors invade thin-walled capillaries and
veins than thick-walled arteries.
V. Metastasis (distant spread)
Metastasis is defined as spread of tumor by invasion in such a way that discontinuous
secondary tumor mass/masses are formed at the site of lodgement. Metastasis is the most important
feature to distinguish malignant from benign tumors. Benign tumors do not metastasize while all the
malignant tumors with a few exceptions like gliomas of the central nervous system and basal cell
carcinoma of the skin, can metastasize.
Routes of metastasis:
1. Lymphatic spread.
2. Hematogenous spread.
3. Other routes (spread along epithelium-lined surfaces, spread via cerebrospinal fluid,
implantation).
1. Lymphatic spread. In general, carcinomas metastasize by lymphatic route while sarcomas favour
hematogenous route. The walls of lymphatics are readily invaded by cancer cells and may form a
continuous growth in the lymphatic channels called lymphatic permeation, or may detach to form
tumour emboli. The tumor emboli enter the lymph node at its convex surface and are lodged in the
subcapsular sinus. Later, of course, the whole lymph node may be replaced and enlarged by the
metastatic tumor. Sometimes lymphatic metastases do not develop first in the lymph node nearest to
53
the tumor because of venous-lymphatic anastomoses or due to obliteration of lymphatics by
inflammation or radiation, so called stop metastasis. Other times, due to obstruction of the lymphatics
by tumor cells, the lymph flow is disturbed and retrograde metastases may be seen at unusual sites, e.g.
metastasis of carcinoma prostate or stomach to the supraclavicular lymph nodes, metastatic deposits
in the adrenals from carcinoma lung etc.
2. Hematogenous spread. Metastasis through blood vessels is the common route for sarcomas but certain
carcinomas also frequently metastasize by this mode, especially those of the lung, breast, thyroid, kidney
and prostate. The common sites for blood-borne metastasis are the liver, lungs, kidneys, brain and
bones, all of which provide “good soil” for the growth of “good seeds” (seed-soil theory) than are the
unfavourable sites like the spleen and muscles. The cancer cells readily invade the walls of capillaries,
venules and veins than the arteries which are thick-walled and contain elastic tissue resistant to
invasion.
Cancers of the organs draining into portal veins frequently establish metastasis in the liver,
while cancers of organs draining into caval veins metastasize to the lungs.
Etiology and pathogenesis of neoplasia
The etiology of tumors is various, 4 theories are recognized.
1. Virogenetic theory. It states integration of the genomes of the virus and the normal cell that is
combination of nucleic acid of the virus with genetic apparatus of the cell, which turns into tumor
ceil. Oncogenic viruses are those containing DNA and RNA (Epstein-barr virus, herpes virus,
hepatitis B- Virus, etc.).
2. Physicochemical theory suggests that tumor appears under the influence of different physical and
chemical substances, so called carcinogens.
3. Dysontogenetic theory was created by J. Cohnheim; According to his theory, tumors appear from
embryonic tissue and abnormally developed tissues under the influence of different causative agents.
4. Polyetiological theory emphasizes the importance of different factors, i.e. chemical, physical, viral,
parasite, dyshormonal.
Based on the current state of knowledge, these factors are broadly described under 2 main
headings:
I. Predisposing epidemiologic factors, which include a number of endogenous host factors and
exogenous environmental factors.
II. Carcinogenesis, that encompasses exogenous agents like chemical, physical, hormonal and
biological substances.
Carcinogenesis
Carcinogenesis means inductions of tumors; agents, which can induce tumors, are called
carcinogens. Carcinogens are a variety of extrinsic agents, which are broadly divided into 4 groups:
1. Chemical carcinogens.
2. Physical carcinogens (mainly radiation).
3. Hormonal carcinogens.
4. Biologic carcinogens (chiefly viruses).
Clinical aspects of neoplasia
Two major aspects of clinical significance in assessing the course and management of
neoplasia are tumor-host inter-relationship and laboratory diagnosis of cancer.
Effect of tumor on host
Malignant tumors produce more ill effects than the benign tumors.
1. Local effects. Both benign and malignant tumors cause local effects on the host due to their size or
location. Some of the local effects of tumors are as under
Compression.
Mechanical obstruction.
Tissue destruction.
Infarction, ulceration, hemorrhage.
2. Cancer cachexia. Patients with advanced and disseminated cancers terminally have asthenia
(emaciation), and anorexia, together referred to as cancer cachexia.
3. Fever. Fever of unexplained origin may be presenting feature in some malignancies such as in
Hodgkin’s disease, adenocarcinoma kidney, osteogenic sarcoma and many other tumors. The exact
mechanism of tumor-associated fever is not known but probably the tumor cells themselves elaborate
54
pyrogens.
4. Paraneoplastic syndromes. Paraneoplastic syndromes (PNS) are a group of conditions developing
in patients with advanced cancer, which are not explained by direct and distant spread of the tumor
(endocrine, neuromuscular, hematologic, gastrointestinal, renal syndromes, amyloidosis).
5. Secondary changes in the tumor result from disturbances of blood circulation, from chemo*-or
radiotherapy. They manifest by foci of necrosis, hemorrhages, inflammation, formation of mucus,
calcification.
Diagnosis of cancer. The most certain and reliable method which has stood the test of time
is the histological examination of biopsy, cytological methods, histochemistry and cytochemistry,
immunohistochemistry.
EPITHELIAL TUMORS
Benign epithelial tumors
Benign epithelial tumors are subdivided according to their origin from different types of
epithelium into the tumors of integumentary epithelium (papillomas), tumors of glandular epithelium
(adenomas).
Papilloma has following features
Bening tumor.
Origin from the skin and mucous membranes.
It looks like a ledge or a bush of branching papillae.
Exophytic tumor.
Slow growth.
The base of the tumor consists of connective tissue containing blood vessels.
It is a continuation of subepithelial connective tissue covered with epithelium like.
May be hard of soft.
Hard papillomas locate on the skin and mucous membranes covered with multilayer
squamous epithelium (mouth, larynx, pharynx).
Soft papillomas consist of thin fibers with thin-walled vessels. They are covered with
cylindrical transition or ciliated epithelium, their thin branching papillae can be easily
injured and bleed. They grow quickly. They often become malignant turning into cancer.
These papillomas are mainly found in the neck of the urinary bladder and in the region of
the triangle.
Adenoma
Benign epithelial tumor from the epithelium of the glands and glandular organs.
More often they can be found in the breast, thyroid gland, liver, ovaries, prostatic gland,
gastrointestinal tract.
According to the histological composition adenoma may be tubular and alveolar.
In tubular adenoma, there are glandular cavities resembling tubes in the connective tissue
with vessels.
In alveolar adenoma, numerous bubbles bedded with cylindrical or cubic epithelium are
observed in the connective tissue with vessels.
Adenomas from compact organs (liver, adrenal gland) can be made of groups of respective
cells separated from each other by a thin layer of stroma.
Thus, the structure of adenomas is similar to that of the original organ, which is the cause of
their functional similarity (ability of adenoma cells to produce respective secretes) e.g. adenomas of mucous membranes - mucus, adenomas of eosinophilic cell of the anterior
lobe of pituitary - somatotropic hormone, medullar layer of adrenal gland - noradrenaline,
beta cells of pancreas - insulin, etc.
Adenomas have atypical structure, which manifests in absence of ducts, variety of shape,
size and location, parenchyma and stroma ratio (fibroadenoma, adenofibroma) in the
glandular tubules and vesicles.
In some adenomas glandular cavities are widened and form large cavities, cysts filled with
serous fluid or mucus. These cyst-like adenomas are called cystoadenomas.
Sometimes epithelial growth is so intensive that the papillae invade the walls of flie cyst,
involve the peritoneum, produce metastases, relapse, cause cachexia and may cause sever
consequences. These adenomas are termed papillary adenocystomas. They develop in
ovaries, thyroid gland. Adenocystomas may become malignant more frequently than the
other adenomas.
Malignant epithelial tumors
55
Immature, or malignant, tumors of epithelium are also called carcinoma. The term came to us
from the time of Hippocrates and Galen.
Precancerous states: defects of development, including lost embryonic germs, chronic
inflammatory diseases, chronic ulcers, disturbed tissue regeneration (abundant granulation,
metaplasia, displasia), hormonal hyperplasias, polyposis of mucous membrane, and leukoplakias of
the mucous membrane.
The morphological classification is based on differentiation of the tumor cells.
According to it all cancers can be divided into 3 groups:
1) Poorly-differentiated: small-cell or large-cell, medullar, scirrhus, solid.
2) Well-differentiated: squamous-cell, with keratinization, without keratinization,
adenocarcinoma (trabecular, alveolar, papillary, mucous.
3) Special kinds: chorionepithelioma, seminoma, hypernephroid cancer.
As to metastases, it is important to know that invasion of the tumor cells in the veins is difficult
because they become narrowed. Blood vessels in the tumors look differently. Usually they have the
structure of capillaries. As a rule, vessels in tumors are new structures but they are connected with
general circulation. The tumors may be connected with the sources of nutrition in different ways. The
more directly they contact, the more intensive is the growth of the tumor, the more rapidly it produces
metastases (e.g., chorionepithelioma, seminoma, hypernephroid cancer).
If both stroma and parenchyma of the tumor are anaplastic, they characterize combination
tumors, termed sarcocarcinomas or carcinosarcomas.
Together with tissue and cellular atypism, malignant tumors are characterized by infiltrating
tumor growth.
Clinical-anatomical practice suggests that tumor, as a rule, does not appear at once, its
development is preceded by different processes characterized by: 1) prolonged chronic course, 2)
association with cell multiplying, 3) failure of conservative treatment.
THE MOST OFTEN TUMORS
Gastric carcinoma
Gastric carcinoma comprises more than 90% of all gastric malignant tumors. The men at
the age of 40-60 suffer more often than women;
Pre-cancer changes in the gastric mucosa: 1. Atrophic gastritis, 2. Adenomatous polyps, 3.
Chronic gastric ulcer.
Gastric carcinoma is most commonly located in the region of gastric canal (prepyloric
region), less common localization are the body, cardia and fundus.
Classification:
According to the deepness of the lesion in the gastric wall there are 2 types of carcinoma:
1. Early gastric carcinoma, when carcinoma confined only mucosa layers. Early gastric carcinoma
must be distinguished from certain related terms: epithelial dysplasia (cellular atypia seen in
intestinal metaplasia such as in atrophic gastritis and pernicious anemia); carcinoma in situ in the
stomach (a state of severe cellular atypia or dysplasia, without invasion across the basement
membrane of the glands).
2. Advanced gastric carcinoma, when it penetrates the muscular layer or beyond. When the
carcinoma crosses the basement membrane into the muscular propria or beyond, it is referred to as
advanced gastric carcinoma. Advanced gastric carcinoma has following patterns:
1) Ulcerative carcinoma. This is the most common pattern. The tumour appears as a
flat, infiltrating and ulcerative growth with irregular necrotic base and raised margin.
It is seen more commonly in the region of gastric canal. Macroscopically, ulcerative
carcinomas are poorly-differentiated adenocarcinomas, which invade deeply into the
stomach wall. Tubular and acinar patterns are seen more commonly.
2) Fungating (polypoid) carcinoma. The second common pattern is a cauliflower
growth projecting into the lumen, similar to what is commonly seen in the large
intestine. It is seen more often in the fundus. The tumor undergoes necrosis and
infection commonly. Microscopically, fungating or polypoid carcinomas are welldifferentiated adenocarcinomas, commonly papillary type.
3) Scirrhous carcinoma. In this pattern, the stomach wall is thickened due to extensive
desmoplasia giving the appearance as “leather bottle stomach” or “linitis plastica”. The
involvement may be localized to pyloric antrum, or diffuse affecting whole of the
stomach from the cardia to pylorus. The lumen of the stomach is reduced. There are no
ulcers but rugae are prominent. Microscopically, it may be an adenocarcinoma or
56
signel-nng cell carcinoma, extensively infiltrating the stomach wall, but due to marked
desmoplasia cancer ceils may be difficult to find.
4) Colloid (mucoid) carcinoma. This pattern is usually seen in the fundus. The tumour
grows like masses having gelatinous appearance due to secretion of large quantities of
mucus. Microscopically, mucoid carcinoma contains abundant pools of mucin in which
are seen a small number of tumor cells, sometimes having signet-ring appearance.
5) Ulcer-cancer. Majority of ulcer-cancers are malignant lesions from the beginning. For
confirmation of cancer in a pre-existing gastric ulcer, the characteristic microscopic
appearance of peptic ulcer should be demonstrable with one portion of the base or the
margin of the ulcer showing carcinomatous changes. Microscopically, ulcer-cancers are
adenocarcinomas without any specific features.
According to the location gastric carcinoma may be:
Pyloric (50%) gastric carcinoma.
Lesser curvature of the stomach (27%).
Cardial gastric carcinoma (15%).
Greater curvature of the stomach (3%).
Fundal gastric carcinoma (2%).
Total gastric carcinoma (3%).
According to the histoiogical signs there are the following types of gastric carcinoma:
Adenocarcinoma: papillary, mucoid, trabecular (well- differentiated).
Signet-ring cell carcinoma, scirrhous carcinoma, solid carcinoma (poorly-differentiated).
Squamous-cell carcinoma.
Adenosquamous carcinoma.
Metastases can be:
1. Lymphogenic. There are 2 types of them: orthograde (with the lymph flow) and retrograde (against
the lymph flow).
In orthograde metastases, they are carried through the lymphatic vessels to regional
lymphatic nodes - along the lesser and greater curvature, around the cardial and
suprapancreatic lymphnodes.
In retrograde metastases they are carried through the lymphatic vessels to the left
supraclavicular lymphnode (Virchow’s gland), ovaries (Krukenberg tumor),
pararectal tissue (Shnitsler’s metastases).
2. Hematogenic metastases are carried with the blood flow to the liver, lungs, brain, bones, kidneys
and adrenal glands.
3. Implantation (contact), when the carcinoma disseminates through the peritoneum or penetrates to
the pancreatic glands.
Carcinoma of lungs
According to the types of growth pulmonary carcinoma may be:
Exophytic (endobronchial) type.
Endophytic (exobronchial and peribronchial) type.
According to the macroscopical signs pulmonory carcinoma may be:
Superficial spreading type.
Polypoid type.
Endobronchial diffusely spreading type.
Nodular type.
Branching type.
Nodular-branching type.
According to the histological types the bronchogenic carcinoma may be:
1. Squamous-cell carcinoma.
2. Adenocarcinoma:
Acinar carcinoma.
Papillary carcinoma.
Bronchiole-alveolar carcinoma.
Solid carcinoma.
3. Small cell carcinoma:
Oat cell carcinoma.
Small cell carcinoma, intermediate cell type.
Combined oat-cell carcinoma.
57
4. Large cell carcinoma.
5. Adenosquamous carcinoma.
According to its location bronchogenic carcinoma may be hilar and peripheral.
Hilar type has following features:
Etiopathogenesis: smoking, atmospheric pollution, occupational causes, dietary factors,
genetic factors, chronic scarring.
The lung cancer arises in the main bronchus or one of its segmental branches in the hilar
parts of the lung.
More often on the right side.
The tumor begins as a small roughened area on the bronchial mucosa at the bifurcation.
As the tumor enlarges, it thickens the bronchial mucosa producing nodular or ulcerated
surface.
Nodular carcinoma grows into a friable spherical mass, 1 to 5 cm in diameter, narrowing
and occluding the lumen.
The cut surface of the tumour is yellowish-white with foci of necrosis and hemorrhages
which may produce cavitary lesions.
It is common to find secondary changes in the lungs such as bronchopneumonia, abscess
formation and bronchiectasis as a result of obstruction and accompanying infections.
The tumor soon spreads within the lungs by direct extension or by lymphatics, and to
distant sites by lymphatic or hematogenous routes.
Peripheral type:
A small proportion of lung cancers, chiefly adenocarcinomas including bronchioloalveolar
carcinomas.
It originates from a small peripheral bronchiole but the exact site of origin may not be
discernible.
The tumor may be a single nodule or multiple nodules in the periphery of the lung
producing pneumonia-like consolidation of a large part of the lung.
The cut surface of the tumor is grayish and mucoid.
Squamous cell (epidermoid) carcinoma
These tumors usually arise in a large bronchus and are prone to massive necrosis and
cavitation.
The tumor is diagnosed microscopically by identification of either intercellular bridges or
keratinization.
Usually the spread of squamous cell carcinoma is more rapid than the other histologic
types.
Frequently, the edge of the growth and the adjoining uninvolved bronchi show squamous
metaplasia, epithelial dysplasia and carcinoma in situ.
Adenocarcinoma of lungs.
Adenocarcinoma is the most common bronchogenic carcinoma in women and is slowgrowing. Adenocarcinoma is further subclassified into 4 types:
1, Acinar adenocarcinoma, which has predominance of glandular structure and often occurs in the
larger bronchi.
2.Papillary adenocarcinoma, which has a pronounced papillary configuration and is frequently
peripherally located in the lungs and is found in relation to pulmonary scars (scar carcinoma).
3. Bronchiole-alveolar carcinoma is characterized by cuboidal to tall columnar and mucus-secreting
epithelial cells growing along the existing alveoli and forming numerous papillary structures.
4. Solid carcinoma is a poorly-differentiated adenocarcinoma lacking acini, tubules or papillae but
having mucus-containing vacuoles in many tumor cells.
Small cell carcinomas.
Small cell carcinomas are frequently hilar or central in location, have strong relationship to
cigarette smoking and are highly malignant tumors. They are most often associated with ectopic
hormone production because of the presence of neurosecretory granules in majority of tumour cells
which are similar to those found in argentaffm or Kulchitsky cells normally found in bronchial
epithelium. Small cell carcinomas may be:
Oat-cell carcinoma is composed of uniform, small cells, larger than lymphocytes with,
dense, round or oval nuclei having diffuse chromatin, inconspicuous nucleoli and very
sparse cytbplasm. These cells are organized into cords, aggregates and ribbons or around
small blood vessels forming pseudorosettes.
58
Small cell carcinoma, intermediate cell type is composed of cells slightly larger than
those of oat cell carcinoma and have similar nuclear characteristics but have more
abundant cytoplasm. These cells are organized into lobules.
Combined oat-cell carcinoma is a tumour in which there is a definite component of
oat cell carcinoma with squamous cell and/or adenocarcinoma.
Large cell carcinoma.
These are undifferentiated carcinomas, which lack the specific features by which they
could be assigned into squamous cell carcinoma or adenocarcinoma.
Large cell carcinomas are more common in men, have strong association with cigarette
smoking and are highly malignant tumors.
The tumor cells have large nuclei, prominent nucleoli, abundant cytbplasm and welldefined cell borders.
Adenosquamous carcinoma.
These are a small proportion of peripheral scar carcinomas having clear evidence of both
keratinisation and glandular differentiation.
Metastases can be:
1. Lymphogenic - through the lymphatic vessels to regional lymphatic nodes -hilar,
mediastinal, cervical, supraclavicular and paraaortic lymphnodes.
2. Hematogenic metastases are carried with the blood flow to the liver, pancreas, brain, bones,
kidneys, adrenal and thyroid glands.
3. Implantation (contact) when the carcinoma disseminates through the pleura or penetrates to
the peribronchial lung tissue.
Secondary complications: hemorrhages, necrosis of the tumor as well as cachexia.
Breast cancer
There are cancer of ducts, parenchyma, nipple and areola.
According to the WHO, carcinoma of the breast is subdivided on 2 main types non-invasive
carcinoma and invasive one.
Non-invasive (in situ) carcinoma
The tumour cells within the ducts or lobules without evidence of invasion. Two types of
carcinoma in situ are described: intraductal carcinoma and lobular carcinoma in situ.
1. Lobular carcinoma in situ is identified only microscopically. In situ lobular carcinoma is
characterized by filling up of terminal ducts and ductules or acini by rather uniform cells, which are
loosely cohesive and have small, rounded nuclei with indistinct cytoplasmic margins.
2. Carcinoma-in situ confined within the larger mammary ducts is called intraductal carcinoma.
Morphological features are:
The tumor initially begins with atypical hyperplasia of ductal epithelium followed by filling
of the duct with tumour cells.
Macroscopically, the tumor may vary from a small poorly-defined focus to 2.5-5.5 cm
diameter mass.
On cut section, tumor shows cystically dilated ducts containing cheesy necrotic material
(comedo pattern), or the intraductal tumour may be polypoid and friable resembling
intraductal papilloma (papillary pattern).
Micrpscopically, the proliferating tumour cells within the ductal lumina may have 4 types
of patterns in different combinations: solid, comedo, papillary and cribriform. They may
occur:
a) Solid type is characterized by filling and plugging of the ductal lumina with
tumorous cells.
b) Comedo type is centrally placed necrotic debris surrounded by neoplastic cells in the
duct.
c) Papillary type has formation of intraductal papillary projection of tumor cells, which
lack a fibrovascular stalk so as to distinguish it from intraductal papilloma.
d) Cribriform type is recognized by neat punched out fenestrations in the intraductal
tumor.
Invasive carcinoma
1. Infiltrating ductal (not otherwise specified) is the classic breast cancer.
59
Macroscopically, the tumor is irregular, 1-5 cm in diameter, hard cartilage-like mass that
cuts with grating sound.
The sectioned surface of the tumor is gray-white to yellowish with chalky streaks and often
extends irregularly into the surrounding fat.
Microscopically, as the name NOS suggests, the tumor is different from other special types
in lacking a regular and uniform pattern throughout the lesion. There are 3 histological
types of this carcinoma:
a) Anaplastic tumor cells forming solid nests, cords, poorly-formed glandular
structures and some intraductal foci.
b) Infiltration by these patterns of tumor cells into diffuse fibrous stroma and fat.
c) Invasion into perivascular and perineural spaces as well as lymphatic and vascular
invasion.
2. Infiltrating (invasive) tubular carcinoma, invasive cancers in being more frequently bilateral and
within the same breast, it may have multicentric origin.
Macroscopically, the appearance varies from a well-defined scirrhous mass to a poorlydefined area of induration that may remain undetected by inspection as well as palpation.
Microscopically, there are 2 characteristics:
a) Pattern - a characteristic single file (Indian file) linear-arrangement of stromal
infiltration by the tumor cells with very little tendency to gland formation is seen.
Infiltrating ceils may be arranged concentrically around ducts in a target-like
pattern.
b) Tumor cytology - individual tumor cells resemble cells of in situ lobula of
carcinoma. They are round and regular with very little pleomorphism and
infrequent mitoses. Some tumors may show signet-ring cells distended with
cytoplasmic mucus.
3. Medullary carcinoma has a significantly better prognosis than the usual infiltrating duct
carcinoma, probably due to good host immune response in the form of lymphoid infiltrate in the
tumour stroma.
Macroscopically, the tumour is characterized by a large, well-circumscribed, rounded mass
that is typically soft and fleshy brain-like and hence the alternative name of “encephaloid
carcinoma.”
Cut section shows areas of hemorrhages and necrosis.
There arc 2 histological characteristics of this tumor:
a) Pleomorphic tumor cells with abundant cytoplasm, large vesicular nuclei and many
bizarre and atypical mitoses are diffusely spread in the scanty stroma.
b) The loose connective tissue stroma is scanty and usually has a prominent lymphoid
infiltrate.
4. Colloid (mucinous) carcinoma contains large amount of extracellular epithelial mucin and acini
filled with mucin. Cuboidal to tall columnar tumour cellsi some showing mucus vacuolation, are seen
floating iti large lakes of mucin.
Paget’s disease of the nipple
The nipple bears a crusted, scaly eczematoid lesion with a palpable subareolar mass in
about half the cases.
Macroscopically, the skin of the nipple and areola is crusted, fissured and ulcerated.
Microscopically, the skin lesion is characterized the presence of Paget’s cells singly or in
small clusters in the epidermis. These cells are larger than the epidermal cells, spherical,
having hyperchromatic nuclei with eytoplasmic halo that stains positively with
mucicarmine.
In these respects, Paget’s cells are adenocarcinoma-type cells. In addition, the underlying
breast contains invasive or non-invasive duct carcinoma, which shows no obvious direct
invasion of the skin of nipple.
The metastases are either local or distant, the former to the lymphatic nodes of the breast
base, axilla, subclavicutar, parasternal nodes. Distant metastases are hematogenic ones, 40
- 50% to the bones, lungs, and liver. Late metastases and relapses occur 5 - 20 years after
the operation.
Tumors cervix and body uterus
60
Cervix is both a sentinel for potentially serious upper genital tract infections and a target for
viral or chemical cancerogens, which may lead to invasive carcinoma.
Squamous cell carcinoma may occur in any age from the second decade of life to senility.
Invasive cervical carcinoma manifests in three somewhat distinctive patterns: fungating (or
exophytic), ulcerating, and infiltrative cancer.
Histologically, about 95% of squamous cell carcinomas are composed of relatively large
cells either keratinizing (well-differentiated) or non-keratinizing (moderately
differentiated) patterns.
Cervical cancer is staged as follows:
1. Carcinoma in situ.
2. Carcinoma confined to the cervix: preclinical carcinoma diagnosed only
microscopically but showing;
3. Carcinoma extends beyond the cervix but not onto the pelvic wall. Carcinoma
involves the vagina, but not to the lower third.
4. Carcinoma has extended onto the pelvic wall. The tumor involves the lower third of
the vagina.
5. Carcinoma has extended beyond the true pelvis or has involved the mucosa of the
bladder or rectum. This stage obviously includes those with metastatic dissemination.
Ten to twenty-five per cent of cervical carcinoma constitutes adenocarcinoma,
adenosquamous arcinoma and undifferentiated carcinoma.
With current methods of treatment, there is % year survival rate about 80 to 90% with stage
1, 75% with stage 2, 35% with stage 3 and 10 to 15% with stage 4 disease.
Carcinoma of endometrium
The uterine corpus including its endometrium and myometrium is affected by a great
variety of neoplastic growth.
These can be benign or malignant and can arise from
1. The endometrium glands (endometrial polyps and endometrial carcinomas).
2. The endometrial stroma (stromal nodule and stromal sarcoma).
3. Mixed mesodermal tumors.
4. The smooth muscle of the myometrium (leiomyoma, leiomyosarcoma).
The most common of these tumors are the endometrial polyps, leiomyomas, and
endometrial carcinomas.
Endomterial polyps are bening tumors.
There are sessile masses of variable size that project into the endometrial cavity. They may
be single or multiple, asymptomatic or may cause abnormal bleeding if the ulcerate or
undergo necrosis.
Histologically, they are generally of two types, made up of
1. Functional endometrium.
2. More commonly hyperplastic endometrium, mostly of the cystic variety.
Rarely, adenocarcinomas may arise within endometrial polyps.
Carcinoma of endometrium
Carcinoma of endometrium is the most common invasive cancer of the female genital tract.
It is uncommon in women younger than 40 years of age. The peak incidence is in the 55 to
65-year-old women.
In terms of potential pathogenesis, two general groups of endometrium cancer can be
identified. The first and the most well-studied develops on a background of prolonged
estrogen stimulation and endometrial hyperplasia.
Grossly, endometrial carcinoma presents as a localized polypoid tumor or as a diffuse
tumor involving the entire endometrial surface.
Eventually dissemination to the regional lymph nodes occurs, and in the late stages, the
tumor may be hematogenously borne to the lungs, bones and other organs.
Histologically, most endometrial carcinoma is adenocarcinomas characterized by more or
less well-differentiated gland patterns lined by malignant stratified columnar epithelial
cells.
The more well-differentiated tumors tend to be those of endometrial differentiation.
61
Squamous elements most commonly are histologically benign in appearance (called
adenocarcinoma with squamous metaplasia or more traditionally, adenoacantoma) when
associated with well-differentiated adenocarcinomas.
Although classification as a poorly differentiated adenocarcinoma typically requires a less of
glandular differentiation and the presence of solid growth, two histologic patterns behave as
poorly differentiated regardless of their degree of differentiation and include papillary
serous carcinoma.
Carcinoma of prostate gland
Cancer of the prostate is the second most common form of cancer in males, followed in
frequency by lung cancer. It is a disease of men above the age of 50 years and its prevalence increases
with increasing age so that 60% or more of men 80 years old have asymptomatic carcinoma of the
prostate.
Precursor for prostatic cancer:
Androgens level is high.
Administration of estrogens.
In patients with Klinefelter’s syndrome.
Racial and geographic influences (in Americans).
Nodular hyperplasia.
There are following 4 types carcinoma of the prostate:
1. Latent carcinoma. This is found unexpectedly as a small focus of carcinoma in the prostate during
autopsy studies in men dying of other causes. Its incidence in autopsies has been variously reported
as 25-35%.
2. Incidental carcinoma. About 15-20% of prostatectomies done.
3. Occult carcinoma. This is the type in which the patient has no symptoms of prostatic carcinoma but
shows evidence of metastases on clinical examination and investigations.
4. Clinical carcinoma. Clinical prostatic carcinoma is the type detected by rectal examination and
other investigations and confirmed by pathologic examination of biopsy of the prostate.
Morphology
Macroscopically, the prostate may be enlarged, normal in size or smaller than normal. In
95% of cases, prostatic carcinoma is located in the peripheral zone, especially in the
posterior lobe. The malignant prostate is firm and fibrous. Cut section is homogeneous and
contains irregular yellowish areas.
Microscopically, there are 4 histologic types of cancer of the prostate adenocarcinoma,
transitional cell carcinoma, squamous cell carcinoma and undifferentiated carcinoma.
Metastases:
Lymphogenous: sacral, iliac and para-aortic lymph nodes.
Hematogenous: pelvis, lumbar spine, lungs, and kidneys, brain.
Liver tumors
Benign tumors
1. Liver cell adenomas are pale, yellow-tan, and frequently bile-stained nodules, found anywhere in
the hepatic substance but often beneath the capsule.
They may reach 30 cm in diameter. Although they are usually well demarcated,
encapsulation may not be macroscopically evident.
Microscopically, liver cell adenomas are composed of sheets and cords of cells that may
resemble normal hepatocytes or have some variation in cell and nuclear size. Portal tracts
are absent; instead prominent arterial vessels and draining veins are distributed through
the substance of the tumor. A capsule that rahges from delicate collapsed reticulin to welldefined connective tissue usually separates the lesion from the surrounding parenchyma,
but it may be deficient in places or entirely absent.
2. Bile duct adenomas are firm, pale, and usually single discrete nodules rarely more than I cm in
diameter, frequently found in a subcapsular location, in contrast to the liver cell adenoma, they are
almost never bile stained.
Microscopically they are composed of uniformly sized, epithelium-lined channels or ducts
separated by a scant-to-abundant connective tissue stroma and sharply demarcated from
the surrounding liver.
Malignant tumors
1. Hepatocellular carcinoma may appear macroscopically as a unifocal (usually large) mass;
multifocal, widely distributed nodules of variable size; or a diffusely infiltrative cancer, permeating
62
widely and some times involving the entire liver.
All three patterns may cause liver enlargement (2000 to 3000 gm), particularly the unifocal
massive and multinodular patterns.
The diffusely infiltrative tumor may blend imperceptibly into a cirrhotic liver background.
When discrete masses can be seen, they are basically yellow-white, punctuated sometimes
by areas of hemorrhage or necrosis.
Hepatocellular carcinoma sometimes takes on a green hue when composed of welldifferentiated hepatocytes capable of secreting bile.
All patterns of hepatocellular carcinoma have a strong propensity for invasion vascular
channels. Hepatocellular carcinoma range from well-differentiated to highly anaplastic
undifferentiated lesions.
In well-differentiated and moderately well-differentiated tumors, cells recognizable as
hepatocytic in origin are disposed either in a trabecular pattern or in an acinar,
pseudoglandular pattern.
Supporting connective tissue is minimal to absent, explaining the soft consistency of most
hepatocellular carcinoma. Bile may occasionally be seen in canalicular spaces or lumens
between tumor cells, and bile canaliculi may be present ultrastructurally.
A distinctive variant of hepatocellular carcinoma is the fibrolamellar carcinoma. This tumor
occurs in young men and women (20 to 40 years of age) with equal incidence, has no
association with HBV or cirrhosis factors, and has a distinctly better prognosis. It usually
constitutes a single large, hard “scirrhous” tumor with fibrous bands coursing through it.
Histologically it is composed of well-differentiated polygonal cells growing in nests or cords
and separated by parallel lamellae of dense collar bundles, hence the name “fibrolamellar.”
2. Cholangiocarcinomas resemble adenocarcinomas arising in other parts of the body.
Cholangiocarcinomas are rarely bile stained because differentiated bile duct epithelium
does not synthesize pigmented bile.
Most are well-differentiated sclerosing adenocarcinomas with clearly defined glandular and
tubular structures lined by somewhat anaplastic cuboidal to low columnar epithelial cells.
These neoplasms are often markedly desmoplastic; so dense collagenous stroma separates
the glandular elements. Mucus is frequently present within cells and the lumina but not
bile.
Tumors of the adrenal medulla
The two principal types of tumour of the adrenal medulla are pheochromocytomas
(occurring in adults) and neuroblastomas (occurring in children).
Pheochromocytoma consists of secreting cells of the adrenal medull. It produces high levels
of adrenaline/noradrenaline hormones and their breakdown products, vanyl mandelic acid
(VMA) and homovanillic acid (HVA), both of which are excreted in the urine and can be
estimated as a diagnostic test.
Macroscopically, the tumor is usually spherical and less than 5 cm in diameter, it has a pale,
creamy cut surface that changes to dark brown almost instantly when exposed to air, due to
oxygenation of tumor pigments. Despite the fact that the tumor is usually small and nonmetastatic, it is a hazardous condition with high perioperative mortality.
The excessive amine production produces hypertension that is often initially paroxysmal
and associated with severe headaches, but the hypertension eventually becomes constant.
There may be intractable, and often unexplained, cardiac failure.
Pheochromocytoma is one of the causes of surgically treatable systemic hypertension.
Thyroid cancer
There are four main types of malignant tumor derived from thyroid follicle cell.
1. The most common type is papillary carcinoma, a well-differentiated tumor that arises most
frequently in young adults. It is often multifocal within the thyroid, and tends to metastasize
via lymphatics to nodes in the neck. It is slow-growing and has an excellent prognosis; even
metastatic tumors grow slowly and can be cured by surgical resection.
2. Follicular carcinoma most commonly affects middle-aged people. Metastasizing via the
bloodstream, it is one of the tumors that characteristically spread to bone. Patients may
occasionally present with a spontaneous fracture due to metastatic disease, before the primary
tumor is detected. It has a good prognosis.
3. Medullary carcinoma is a less frequent derived from parafolicular or C-cells and characterised
by fibrovascular septa and amyloid-conteining stroma, irregular calcification, atypical cells.
63
4. Entirely confined to the elderly, anaplastic carcinoma grows very rapidly, extensively invading
tissues near the thyroid, such as the trachea and soft tissues of the neck. It may present with a
rapidly enlarging thyroid mass causing tracheal compression or jugular vein invasion. The
prognosis is very poor. The cells of the tumor, which are usually small, undifferentiated and
round, must be distinguished histologically from malignant lymphoma; the latter can also
affect the thyroid in the elderly, but is more responsive to treatment.
Pancreatic islet cell tumors
Islet cell tumors may be hormonally inactive or may produce hyperfunction.
These tumors make one or more of the following: glucagon, insulin, gastrin, somatostatin,
vasoactive intestinal polypeptide (VIP), pancreatic polypeptide (PP).
The tumors are usually slow-growing, even when malignant (10% of total, higher for
glucagonomas, gastrinomas, ACTH-omas and somatostatinomas).
The surrounding pancreatic islets may show hyperplasia,
Metastatic spread remains the only reliable criterion for malignancy in these tumors.
They are named according to their histogenesis:
Beta cell tumors ("insulinomas")
the most common islet cell tumors (* but their incidence is only about 1 in 1,000,000
people)
Look for Whipple's triad:
1. low measured plasma glucose (hypoglycemia);
2. mental changes, especially related to fasting or exercise;
3. attacks relieved by glucose administration.
Often, patients become massively obese.
The etiology of these tumors is obscure; there's not even an important genetic syndrome.
Of beta-cell tumor patients, 70% have a solitary adenoma, while the rest have either
hyperplasia of many islands, or a beta cell carcinoma.
Grossly: usually solitary and well-encapsulated. Size from0/5 to 10cm.
Microscopically: cords and sheets of well-differentiated B-cells which do not differ from
normal cells.
Many mesotheliomas and retroperitoneal fibrosarcomas, and occasionally other tumors,
produce an insulin-like activity (probably somatomedin, but it varies).
Gastrinomas (Zollinger-Ellison Syndrome; "G-cell tumors")
An especially troublesome syndrome of multiple bleeding ulcers and diarrhea. The majority
of gastrinomas are low-grade malignancies. May be benign and malignant.
Gastrinomas occur in the wall of the duodenum.
High basal acid secretion plus a marked increased in serum gastrin levels in response to
secretin administration strongly suggests gastrinoma.
Glucagonomas ("alpha-two cell tumors"). These produce mild diabetes, sore tongue,
and necrolytic migratory erythema. Don't miss this diagnosis.
Delta cell tumors ("somatostatinomas"): diabetes, diarrhea, gallstones, etc.
Tumors secreting vasoactive intestinal polypeptide ("VIPomas"; "VernerMorrison syndrome"): pancreatic cholera (horrible diarrhea), loss of potassium, achlorhydria -excellent response to somatostatins.
PP-omas ("P-cell tumors"): no syndrome despite huge amounts of pancreatic polypeptide
Multiple Endocrine Neoplasia Syndromes ("MEN", formerly "MEA", adenomas):
some or all of the following in same family:
Wermer's MEN I: pituitary adenoma, parathyroid adenoma, Zollinger-Ellison
Sipple's MEN II(a): Parathyroid adenoma, pheochromocytoma, medullary carcinoma of the
thyroid
MEN IIb/III: Medullary carcinoma of the thyroid, pheochromocytoma, mucosal neuromas,
Marfanoid habitus.
MESENCHYMAL TUMORS
In ontogenesis, mesenchyma gives the beginning to
1) connective tissue,
2) vessels,
3) muscles,
4) tissues of musculoskeletal system,
64
5) serous membranes,
6) hemopoietic system.
Mesenchymal tumors develop from
1) connective (fibrous) tissue,
2) fat tissue,
3) muscular tissue,
4) blood and lymphatic vessels,
5) synovial tissue,
6) mesothelial tissue,
7) bone tissue.
They may be benign (name of the tissue + oma) and malignant (name of the tissue + sarcoma).
There are also special terms (e.g. desmoid, granular-cell tumor).
Connective (fibrous) tissue tumors
Main benign connective (fibrous) tissue tumors
1) Fibroma - a node of differentiated connective tissue with different direction of the bands:
Dense -fibrous structures prevail over the cellular elements.
Soft (loose connective tissue with great amount of stroma cells -fibroblasts and fibrocytes).
Localization of fibroma is various: skin, breast.
2) Desmoid fibroma is a kind of dense fibroma and characterized by infiltrating growth and relapses.
It is composed of banal, “tame-looking” fibroblasts that do not metastasize. More often it is located on
the anterior abdominal wall.
3) Dermatofibroma (histiocytoma) - small fibrous node with yellow-brown color. More often it is
located in the skin of the legs. Histologically, dermatofibromas are composed of intertwined and
anastomosing bundles of fibroblasts surrounded by dense collagen. There are giant polynuclear cells
with lipids and hemosiderin between cells.
Malignant connective (fibrous) tissue tumors
Macroscopically sarcoma looks like “fish flesh”. As a rule sarcoma metastases are disseminated
hematogenically. Tumors are characterized by atypical cells, including loss of the structure.
1) Fibrosarcoma occurs in deep soft tissue sites, showing increased fibroblastic cells, anaplasia, and
abundant mitotic figures. It looks like node or encapsulated formation. There are 3 types of
fibrosarcoma:
Differentiated fibrosarcoma is characterized by prevalence of fibrous component over
cellular component.
Poorly differentiated fibrosarcoma, termed cellular sarcoma. It is characterized by
prevalence of cellular component over fibrous component. It produces metastases more
frequently.
Round-cell tumors of unknown origin, termed unclassified tumor.
2) Dermatofibrosarcoma protruberans (malignant histiocytoma)
It is essentially a well-differentiated, slow-growing fibrosarcoma of the skin. It is locally
aggressive but rarely metastasizes.
Grossly, they are multilobulated gray-white, fleshy, infiltrative, and unencapsulated but
deceptively circumscribed.
Microscopically, there are cellular neoplasm composed of radially oriented (“storiform”)
fibroblasts, showing spindled and polygonal cells; mitoses are not as numerous as in
fibrosarcoma.
The overlying epidermis is thinned and there often is microscopic extension into
subcutaneous fat.
Tumors of fatty tissue
Benign:
1. Lipoma
It is the most frequent soft tissue tumors, arising in subcutaneous regions at any site but
most commonly on the back, shoulder, and neck.
They can also arise in the mediastinum, retroperitoneum, or bowel wall.
It may develop in every site where there is fat tissue.
Lipomas are encapsulated, usually small yellow node.
65
Intramuscular infiltrating lipoma is a tumor without distinct (clear) borders, it infiltrates to
intermuscular connective tissue.
2. Hybernoma is a rare tumor of brown fat and consists of large round cells with granular or foamy
cytoplasm (fat vacuoles).
Malignant:
1. Liposarcoma (lipoblastic myoma) is a rare, large tumor, which is composing of lipocytes of
different degree of maturity and lipoblasts. There are several types of liposarcoma:
a) Well differentiated (lipoma-like) liposarcoma.
b) Myxoid (embryonic) liposarcoma tend to be low-grade tumors, which are stubbornly
recurrent, follow a more protracted course, and metastasize late.
c) Round-cell liposarcoma.
d) Pleomorphic liposarcoma are high-grade, aggressive sarcomas (85-90% metastasize).
2. Malignant hybernoma is a very rare tumor with cellular polymorphism and a lot of giant cells.
Tumors of the muscles
Benign:
1. Leiomyoma
Leiomyoma consists of smooth muscle with chaotic (confused) location of the muscular
tissue bands, the stroma with vessels and nerves.
If stroma prevails this tumor is termed fibromyoma.
Leiomyomas occur predominantly in the female genital tract, but they may also occur at
other body sites where smooth muscle is well represented (scrotum, nipple, bowel wall).
Secondary changes: necrosis, hemorrhages, cysts, hyalinosis, petrifaction are characterized
for leiomyomas.
2. Rhabdomyoma
Rhabdomyoma consists of striated muscles. It resembles embryonic muscular fibers and
myoblasts.
It appears against a background of tissue shifts and is accompanied by other development
defects (large masses of striated muscles).
The tumors are most frequently primary tumor of the heart in infants and children and are
frequently discovered in the first years of life because it leads to obstruction of valve orifice
or cardiac chamber.
3. Granular-cell tumor (Abrikosov's tumor): this is small tumor in a capsule. It is located in the
tongue, esophagus, and skin. The cells have round shape, large with granular cytoplasm (no lipids).
Malignant:
1. Leiomyosarcoma (malignant leiomyoma) with cellular and tissue atypism, a large number of
mitoses (high mitotic index) are characteristic. These rare tumors arise in the skin, deep soft tissues,
the stomach and particularly in the uterus from pre-existing myomas.
2. Rhabdomyosarcoma (malignant rhabdomyoma) is a more common, especially in children, in the
head and neck and urogenital region. It is subdivided into four types based on the morphologic
features:
The pleomorphic type occurs in patients over 45 years. This variant has large, atypical
tumor cells, some showing abundant cytoplasm with cross striations characteristic of
skeletal muscle differentiation.
Embryonal, botryoid, alveolar types are poorly differentiated tumors of small blue cells that
have focal skeletal muscle differentiation (rhabdomyoblasts with abundant eosinophilic
cytoplasm or cross striations). The botryoid pattern is basically a morphologic variant of
embryonal, with grape-like masses projecting into a cavity such as: vagina, bladder. These
tumors may be apparent only with immunohistochemical investigation.
3. Malignant granular-cell tumor (malignant myoblastoma) resembles malignant rhabdomyoma but
the cytoplasm is granular.
Tumors of synovial tissue
1. Benign synovioma develops in the tendons and tendon sheath. It contains a lot of stroma with
hyalinosis, and a little number of vessels. There may occur xanthomic cells and clefts.
2. Synovial sarcoma (malignant synovioma) develops around the large joints (but not in joint spaces),
in the parapharyngeal region, in the abdominal wall, and less commonly at other body sites. It has
66
polymorphic structure. Some tumors have polymorphic cells and pseudoepithelial gland formations
with cysts, the other have fibroblastoid atypical cells and collagen fibers, structures resembling
tendons.
Tumors of mesothelial tissue
1. Benign mesothelioma resembles a dense node in serous membrane (pleura); its structure is similar
to fibroma (fibroid mesothelioma).
2. Malignant mesothelioma (peritoneum, pleura, pericardium) microscopically looks like atypical
large cells with vacuolized cytoplasm. Malignant mesothelioma may has tubular and papillary
structures. Mesothelioma with tubular and papillary structures is called epithelial mesothelioma.
Tumors from blood and lymph vessels
Benign tumors from blood vessels and lymph vessels
1. Hemangioma is a tumor from blood vessels. There are several types of hemangioma:
a) Capillary, which develops in the skin, mucous membranes, gastrointestinal tract, liver, more
often in children. Capillary hemangiomas are well-defined but unencapsulated lobules. These
lobules are composed of capillary-sized, thin-walled, blood-filled vessels. These vessels are
lined by single layer of plump endothelial cells surrouded by a layer of pericytes.
b) Cavernous hemangioma locates in the liver, skin, bones, muscles, gastrointestinal tract, brain.
They are single or multiple, discrete of diffuse, red to blue, soft and spongy masses. They are
often 1 to 2 cm in diameter. Cavernous hemangiomas are composed of thin-walled cavernous
vascular spaces, filled partly or completely with blood.
2. Glomus tumor (glomus angioma). Glomus tumor is an uncommon true benign tumor arising from
contractile glomus cells that are present in the arteriovenous shunts. These tumors are found most
often in dermis of the fingers or toes under a nail. These lesions are characterized by extreme pain.
Microscopically: the tumors are composed of small blood vessels lined by endothelium and
surrounded by aggregates of glomus cells. The glomus cells are round to cuboidal cells with scanty
cytoplasm. The intervening connective tissue stroma contains some non-myelinated nerve fibres.
3. Lymphangiomas growth of lymphatic vessels in different direction with formation of a node or
enlargement of the organ. If lymphangioma develops in the tongue, it is termed macroglossia; if
lymphangioma develops in the lip it is termed macrocheilia.
a) Capillary lymphangioma is small, circumscribed, slightly elevated lesion measuring 1 to 2 cm
in diameter. It is composed of a network of endothelium-lined, capillary-sized spaces
containing lymph and often separated by lymphoid aggregates.
b) Cavernous lymphangioma is more common than capillary type. It consists of large dilated
lymphatic spaces lined by flattened endothelial cells and containing lymph. A large cystic
variety called cystic hydroma occurs in the neck producing gross deformity.
Malignant tumors from blood vessels and lymph vessels
1. Angiosarcoma also known as hemangiosarcoma and malignant hemangioendothelioma, it is a
malignant vascular tumor occurring in the skin, subcutaneous tissue, liver, spleen, lung and
retroperitoneal tissues. The tumor is usually well-defined, gray-red, polypoid mass. The tumors may
be well-differentiated masses of proliferating endothelial cells around well-formed vascular channels,
to poorly-differentiated lesions composed of plump, anaplastic and pleomorphic cells in solid clusters
with poorly identifiable vascular channels.
2. Malignant hemangiopericytoma is a tumor arising from pericytes. Pericytes are cells present
external to the endothelial cells of capillaries and venules. The tumor is composed of capillaries
surrounded by spindle-shaped pericytes outside the capillary basement mambrane forming whorled
arrangement. Silver impregnation stains are employed to confirm the presence of pericytes outside
the basement mambrane of capillaries and to distinguish it from hemangioendothelioma. These
tumors are highly malignant with early metastases in skin, liver, and muscles.
3. Kaposi’s sarcoma is a malignant angiomatous tumor, first described by Kaposi, Hungarioan
dermatologist, in 1872. However, the tumor has attracted greater attention more recently due to its
frequent occurrence in patients with AIDS. Presently, four forms of Kaposi’s sarcoma are described:
Classic (european) Kaposi’s sarcoma. Thedisease is slow and appears as multiple, small,
purple, dome-shaped nodules or plaques in the skin, especially on the legs.
67
African (Endemic) Kaposi’s sarcoma. It is found in younger age and has a more aggressive
course than the classic form. The disease begins in the skin but grows rapidly to involve
other tissues, especially lymph nodes and the gut.
Epidemic (AIDS-associated) Kaposi’s sarcoma. The cutaneus lesions are not localized to
lower legs but are more extensively distributed involving mucous membraines, lymph
nodes and internal organs early in the course of disease.
Kaposi’s sarcoma in renal transplant cases. This form is associated with recipients of renal
transplants who have been administered immunosuppressive therapy for a long time. The
lesions may be localized to the skin or may have widespread systemic involvement.
Microscopically, the changes are nonspecific in the early patch stage and more characteristic in
the late nodular stage:
In the early patch stage, there are irregular vascular spaces separated by interstitial
inflammatory cells and extravasated blood and hemosiderin.
In the late nodular stage, there are slit-like vascular spaces containing red blood cells and
separated by spindle-shaped, plump tumor cells. These spindle-shaped tumor cells are
probably of endothelial origin.
4. Lymphangiosarcoma is seldom tumor and appears as a result of chronic lymphatic stasis.
Bone tumors
Benign:
1. Osteoma composes of mature compact bone and develops as a rule in spongy and tubular
bones, skull. 2 types of osteoma are known: a) spongy osteoma, b) compact osteoma.
2. Osteoid-osteoma is a small, usually about 1 cm in diameter, and is most often located in the
cortex near the ends of the tibia and femur. Histologically, the osteoid is lined by osteoblasts and
surrounded by fibrous tissue.
3. Benign osteoblastoma (giant osteoid osteoma) is larger than osteoma (greater than 2 cm),
it tends to be located in the vertebrae or long bones, and does not cause as much pain.
4. Giant cell tumor of bone (osteoblastoclastoma) is mostly benign but locally aggressive
tumors that tend to recur if not removed completely. It most found in the epiphyseal ends of long
bones in adults between 20-55 years of age. The histologic pattern is one of uniformly distributed,
osteoclast-like, multinucleated giant cells in a plamp spindle-cell background. There may be foci of
necrosis, hemorrhage, hemosiderin, and/or osteoid. In about 10% of cases, the tumors are composed
of highly atypical fibroblastic cells and are, thus, classified as sarcomas.
Malignant:
Osteosarcomas (osteogenic sarcoma) compose of bone-forming cells. They locate in the
medullary cavity of the metaphyseal ends of long bones. They present as gray-white, invasive, and
destructive masses showing focal hemorrgage and necrosis. Some are largely fibroblastic, others
largely osteoblastic, some chondroid, and others highly vascular (telangiectatic). All form osteoid and
/or bone-incorporating malignant cells. Two types of osteosarcoma are known:
1. Osteoblastic type (bone formation).
2. Osteolytic type (bone destruction).
Metastasize widely, usually to lung first but also to other organs and bones (lymph node
metastases are rare).
Cartilage tumors
Benign:
1. Chondroma derives from hyaline cartilage in the feet, spine, breastbone, and pelvis. If tumor is
located in the peripheral area of the bone it is termed ecchondroma. If in the center area of the bone,
and, like exostoses, it is termed enchondroma.
2. Benign chondroblastoma (Godman’s tumor) consists of chondroblasts, interstitial substance,
marked osteoclast reaction. It found in epiphyses. Resembling embryonic chondroblasts, tumor cells
are polygonal, arranged in sheets, and sometimes surrounded by a lacelike pattern of calcification.
Their nuclei are often deeply indented or longitudinally grooved. Multinucleated, osteoclast-like
giant cells may be present and abundant enough to suggest giant cell tumor of bone. The vast
majority is benign, but rare examples have metastasized to lung.
Malignant:
68
Chondrosarcomas are malignant tumor consisting of cartilage.
It may be primary and secondary. Primary chondrosarcoma originates in bones the trunk
(pelvis, vertebrae, ribs) and adjacent proximal ends of long bones of the extremities.
Secondary chondrosarcoma develops from pre- existing chondromas.
Depending on their location, there are peripheral and central chondrosarcomas.
The tumor tissues have a graish-white, glassy consistency.
There are also characteristic, irregular, partly mucinous, partly hemorrhagic areas of
necrosis as well as pseudocysts.
The cortex is breached in several areas, and the spongiosa is invaded.
Microscopically: atypical cartilage with plump nuclei; frequent multinucleated cells,
pleomorphism, two or more cells in the lacunae.
TUMORS OF NERVOUS SYSTEM AND BRAIN MEMBRANES
Tumors of nervous system develop from different elements of the nervous system:
1. Central.
2. Vegetative.
3. Peripheral.
4. Mesenchymal elements, which are also a part of this system.
Astrocyte tumors or gliomas
The most frequent brain tumors.
They develop from astrocytes and can be found in all brain portions.
The highest incidence is observed between the ages 25 - 45.
The diameter of the tumor is about 5-10 cm. They do not always have distinct boundaries
with the surrounding tissue. It is homogeneous on incision. As a rule, considerable
enlargement of the brain portions is observed.
Astrocytoma is characterized by cyst formation (one or several). They contain colloid
substance or yellowish fluid with large amount of protein.
There are three histological types of astrocytoma:
1. Fibrillar tumor is rich in glial fibers looking like parallel bands, it contains small
amount of astrocytes.
2. Protoplasmatic astrocytoma consists of different in size cells with processes,
which resemble astrocytes; their processes form thick interlacing.
3. Fibrillar-protoplasmatic tumor is characterized by even location of astrocytes
and glial cells.
Cerebellar astrocytoma and subependymal astrocytoma are separate subtypes. A malignant
type is astroblastoma characterized by rapid growth, polymorphism and necroses in the
tumor. This tumor is rare, it disseminates through the liquor routs.
Oligodendroglial tumors
In the majority of cases these are benign.
The highest incidence is observed at the age of 30 - 40. In rare cases, they occur in children.
They are mainly localized in the large hemispheres of the brain, more seldom in the region
of visual tuber and in the trunk. Very seldom, they develop in the area of cerebellum and
spinal cord. Primary multiple oligodendrogliomas of meninges and visual nerves were also
described.
Macroscopically, the tumor is pinkish-gray, resembles brain substance and is diagnosed by
the enlargement of the brain portion. Its consistency may be paste-like; when calcifications
are present it may be dense.
Microscopically it consists of homogeneous small cells with round nuclei and narrow
outline of cytoplasm, which is poorly colored. Sometimes it is characterized by the structure
resembling honeycombs. The tumor is usually poor in vessels. Hyalinosis and calcification
may also be observed.
The types of oligodendrogliomas are fusiform cell and polymorphocellular.
A malignant type of the tumor is oligodendroglioblastoma characterized by special cell
location, marked polymorphism with giant cells. It is also characterized by numerous
mitoses and necrosis foci. The metastases spread through the liquor routs, more often along
the walls of the ventricles. Symmetrical location of the tumor nodes in the walls of the
ventricles is typical.
69
Ependymal tumors and tumors of choroid epithelium
Three types of ependymal tumors are distinguished.
1) Ependymoma (glioma connected with ventricular ependymoma) looks like intra- or
extraventricular node.
The foci of necrosis and cysts can be found in it
Clasters of uni- and bipolar cells around the vessels (so-called pseudorosettes) and cavities
covered with epithelium (true rosettes) are typical.
Most frequently they are located in caudal portions of rhomboid fossa. Ependymoma may
go down the spinal canal (craniospinal tumors).
Ependymoma is usually localized in the bed of the 4th ventricle and in the 3th ventricle.
In the area of the spinal cord, they first grow intramedullary, and then become
extramedullar.
Macroscopically they look like nodes of different size with tuberous (4th ventricle) or
villous (lateral ventricle) surface. The color is pinkish-gray; the consistency is soft.
Microscopic study reveals perivascular structures of radially located cells. Their processes
form a fibrous ring between the body of the cell and the wall of the vessel and over the body
of the cell. In the rest of the tumor tissue, the cells are located in mosaic manner. Single and
multiple clefts and tubes bedded with cylindrical epithelium are common.
2) Ependymoblastoma is a malignant type of ependymoma. This is characterized by marked cellular
polymorphism. It grows quickly, metastases spread through the liquor system. Dedifferentiated
ependymoma is a transitory form between the two types: Choroid papilloma, Choroid carcinoma.
1. Choroid papilloma is a tumor from the epithelium of vascular plexus, looking like a
villous node in the cavity of the brain ventricle.
It is mainly observed in young people.
It consists of numerous, villous structures of cubic or prismatic epithelial cells. It is located
within the brain ventricles. Heterotopic types are rare (horse’s tail).
Macroscopic study demonstrates well-outlined nodes of various sizes. The surface of the
tumor is small- or large-villous, has cauliflower- or mulberry-like appearance. Consistency
is either dense or soft; the color is pinkish-gray.
Microscopically it consists of villi; their connective-tissue stroma is covered with cubic or
cylindrical epithelium. Hyalinosis can be frequently observed.
2. Choroid carcinoma is a malignant type of choroidpapilloma. It is made of anaplastic
cells covering the vascular plexus. Papillary cancer is a rare tumor.
3) Neuronal tumors:
Ganglioneuroma is a rare mature tumor. Most frequently it is localized in the bed of the
3th ventricle, seldom in the hemispheres of the brain. It usually occurs in children and
juveniles. The tumor consists of mature ganglionic cells divided with the bands of glial
stroma. Macroscopically ganglioneuroma looks like a limited node. In the medulla
oblongata it is diffuse, in the cerebellum it looks like hyperplastic folds.
Cerebellum ganglioma is characterized by proliferation of large nervous elements of
Purkinier's cell type.
Ganglioneuroblastoma is a malignant analogue of ganglioneuroma (malignant
gangliocytoma). This is an extremely rare tumor of CNS. It is characterized by cellular
polymorphism and similar to malignant glioma.
Neuroblastoma is a rare highly malignant brain tumor. It occurs mainly in children. The
tumor is formed from large cells with bubble-like nucleus. Mitoses are numerous. The cells
grow like sincitium. There are a lot of vessels.
Poorly differentiated and embryonic tumors
Medulloblastoma and glioblastoma belong to this group. The latter occurs in children.
1. Medulloblastoma is a tumor made by immature cells, medulloblasts; therefore it is highly
malignant.
The most frequent idealization is vermis cerebelli.
Macrpscopically, it is pinkish-gray.
Microscopically medulloblastoma consists of homogenous small cells with dark round or
oval nucleus and poorly seen rim of cytoplasm. The cells are located close to each other.
Rosette is typical. Mitoses are numerous. Vessels are not numerous.
Metastases spread through the liquor routs.
2. Glioblastoma is the second (after astrocytoma) in the incidence. Synonyms: multiform
glioblastoma, glioblastoma, spongioblastoma.
70
It occurs at the age of 40 - 60.
It is situated in the white substance of the brain. This tumor is mainly located in the large
hemispheres of the brain, sometimes in the trunk.
It is characterized by rapid infiltrative growth without distinct boundaries.
Glioblastoma usually produces regional metastases; those to the inner organs are rare
(lungs).
Macroscopically it is motley-colored due to necroses and hemorrhages.
Microscopically the tumor is characterized by marked polymorphism. The cells are located
disorderly (cellular chaos), their size and shape are various, from small lymphocyte-like to
giant polynuclear. Necroses, hemorrhages and vascular growths are typical. Mitoses and
centers of atypical division are frequent.
Meningovascular tumors
These tumors appear from the meninges. The most frequent is meningioma and its malignant
variant meningeal sarcoma.
1. Arachnoidendothelioma (meningioma)
Arachnoidendothelioma (meningioma) is the most frequent type of meningovascular
tumors. They mainly occur in adults over 30, while in children, they are rare. It is also
termed psammoma.
They are characterized by slow expansive growth.
Arachnoendothelioma is usually localized in I) longitudinal sinus and Paccionian bodies, 2)
convex, 3) falciform process, 4) olfactory region, 5) wings and body of main bone, 6)
tubercle of the saddle, 7) the region of semilunar node of trigeminal nerve, 8) tentorium
cerebelli, 9) vascular plexi.
Macroscopically, arachnoidendothelioma looks like well-limited solitary (in rare cases,
multiple) nodes; their consistency is dense, elastic. The tumor is on incision they are
grayish-pink with light bands.
Microscopically large endothelium-like cells characterize it. The cells usually form groups
(plate-like, curl-like, band-like), so-called endotheliomatous structures. In these tumors,
there are secondary changes (calcifications, psammoma bodies, paste).
Types of arachnoidendotheliomas:
1. Endotheliomatous.
2. Fibrous arachnoidendothelioma with plenty of connective tissue fibers.
3. Meningotheliomatous characterized by microcircular structures.
4. Alveolar.
5. Xantomatous.
2. Meningeal sarcoma is malignant type of the tumor.
Histologically it resembles fibrosarcoma, polymorphocellular sarcoma, and diffuse
sarcomatosis of the meninges.
Thus, morphogenetic variety of CNS tumors, difficult diagnosis and differential diagnosis as
well as their localization allow including them into a separate group.
Special attention should be paid to development of secondary signs, which appear due to
the influence on the craniobasal and distal regions of the brain.
Secondary syndromes are dislocation syndromes which are dangerous for the life of the
patient; entrance of the temporal lobe to the tentorial foramen with strangulation of the
midbrain; vasomotor vascular crises, heart failure; wedging of cerebellum tonsil to the great
foramen; regional foci of circulation disturbance (insults and hemorrhages); epileptiform
attacks.
Only correct diagnosis helps to determine the tactics of treatment for such patients.
Tumors of vegetative nervous system
Tumors of vegetative nervous system originate from ganglionic cells of different degree of
maturity (sympathogonias, sympathoblasts, ganglioneurocytes) in sympathetic ganglia as well as from
the cells of nonchromophinic paraganglia (glomes) genetically connected with sympathetic nervous
system.
Such benign tumors as ganglioneuroma, paraganglioma (glomes tumor, chemodektoma)
belong to this group.
Ganglioneuromas are localized in the medullar substance of the adrenal gland,
sympathetic trunks, cerebrospinal nerves. It usually develops in young patients. The tumor
71
differs from normal ganglia as it has the signs of atypism (polynuclear cells, tigrolysis, and
nuclear decentralization. Schwann’s glia is represented by satellite cells. The tumor does not
produce metastases.
Malignant ganglioncuroblastoma is a combination of neuroblastoma and
ganglioneuroma. The tumor develops intrauterinely or during the first years of life. It may
be localized in any region of vegetative nervous system, small intramural ganglia of the
inner organs, medullar layer of adrenal glands and sympathetic trunks. Sometimes it
matures and turns into ganglioneuroma.
Nonchromophilic paraganglioma is a benign variant. It resembles the tumors of
APUD system (APUDomas). It can produce ACTH and serotonin. The tumor is localized in
the middle ear, retroperitoneally. It may be large; its histological structure is alveolar and
trabecular with large number of sinusoid vessels.
Malignant paraganglioma. This is characterized by cellular polymorphism, infiltrating
growth, lymphogenic metastases. Thus, the tumors of peripheral ganglias correspond to
different stages of their embryonic structure. Lest mature is neuroblastoma, the most
manure is ganglioneuroma. Ganglioneuroblastoma occupies an intermediate place.
Tumors of peripheral nervous system
Tumors of peripheral nervous system originate from the nerve membranes. Neurilemma
(Schwannoma), neurofibroma, neurofibromatosis (Recklinghausen’s disease) are benign ones.
Schwannoma is formed of spinder-like cells with rod-shaped nuclei. The cells and fibers
form rhythmical structures. Neurofibroma is a tumor connected with the nerve membrane.
It consists of connective tissue with nervous cells, bodies and fibers.
Neurofibromatosis is a systemic disorder characterized by development of multiplies
neurofibromas associated with different development defects. This may be peripheral and
central.
Malignant neurilemma is neurogenic sarcoma. Pilymorphocellular atypism, polynuclear
symplasts, garden-like structure is characteristic.
TUMORS OF MELANIN-PRODUCING TISSUE
Melanin-producing cells (melaninocytes) are of neurogeneous origin. They may become the
origin of tumor-like formations (nevi) and melanomas.
Nevi are benign tumors of skin consisting of melanocytes of epidermis and derma.
Neurogeneous origin of melanocytes is generally recognized. Nevi are defects of
development of neuroectodermal pigment elements.
They look like brown spots of different size, and may be either flat or elevated over the
surface or be wart-like. Sometimes their size is enormous (giant pigmented nevus).
According to the WHO classification (1974), there are the following types of nevi:
1. Junctional nevus.
2. Compound nevus.
3. Intradermal.
4. Epithelioid nevus (intracellular).
5. Balloon-cell nevus.
6. Halo nevus.
7. Giant pigmented nevus.
8. Involution nevus (fibrous papule of the nose).
9. Blue nevus.
10. Cellular blue nevus.
Junctional nevus. Nests of nevus cells are found on the border of epidermis and dermis. The
nests are round or oval. Their-cytoplasm is homogeneous, slightly granular. The nevus cells are
localized in the area of reticular layer apices.
Compound nevus. Together with the nevus cells located on the border of dermis and
epidermis, there are nests of nevus cells in derma itself.
Intradermal nevus. Nevus cells are located only in derma. Some of them can be found on
the border between derma and epidermis. They resemble nests. The nevus cells look like compact
mass. The cells in mature nevi may be polynuclear. Macroscopically they have papillomatous
appearance and may contain hairs.
Epithelioid nevus or juvenile melanoma can often appear on the face, especially in
children. It looks like flat or ball-like node. The surface of the skin is smooth, sometimes-
72
papillomatous changes are observed. Microscopically it looks like compound nevus with borderline
changes. Sometimes marked acanthosis is present. The amount of melanin is small; it may also be
absent. The cells have light basophilic cytoplasm and hyperchronic nuclei. Epithelioid cells with large
foamy light cytoplasm may be present. Mitoses are not numerous. Uni- or polynuclear cells resemble
Teuton’s cells. There are a lot of vessels.
Blue nevus. Macroscopically this looks like bluish or bluish-brown or bluish-gray sport, its
shape is round or oval, it does not elevate over the surface of the skin. Microscopic examination
reveals stretched melanocytes.
Melanoma
Melanoma is one of the most malignant tumors, it spreads through the lymphatic and
hematogenic routs. 70% of melanomas develop on the skin of the face, body and extremities.
Depending upon the clinical course and prognosis, cutaneus malignant melanomas are of the
following 4 types:
1. Lentigo maligna melanoma. This often develops from a pre-existing lentigo. It is
essentially a malignant melanoma in situ.
2. Superficially disseminated melanoma (invasive melanoma). This is a slightly
elevated lesion with variegated color and ulcerated surface. It often develops from a
superficial spreading melanoma in situ. Melanomas may not contain pigments. In the
tumor, there are a lot of mitoses, hemorrhages and necroses. The tumors are localized
on the skin, pigment membrane of the eye, meninges, and medullar layer of adrenal
glands, in rare cases mucous membranes.
3. Acral lentigenous melanoma. This occurs more commonly on the soles, palms and
mucosal surfaces. The tumor often undergoes ulceration an early metastases.
4. Nodular melanoma. This often appears as an elevated and deeply pigmented nodule
that grows rapidly and undergoes ulceration. This variant carries the worst prognosis.
Histologically, melanoma cells are larger than nevus cells with irregular nuclei and prominent
eosinophilic nucleoli; they grow as loose nests lacking the typical features of maturation. They may be
epithelioid or spindle-shaped. Mitotic figures are often present and multinucleate giant cells may
occur. These tumor cells may be arranged in various patterns such as solid masses, sheets, island,
alveoli etc. Melanin pigment may be present or absent without any prognostic influence. Some
amount of inflammatory infiltrate is present in the invasive melanomas. Although morphologic
variants of the radial growth phase have been described, the nature and extent of the vertical growth
phase determines the biologic behavior and prognosis.
Metastatic spread of melanoma is very common and takes place via lymphatics to the regional
lymph nodes and through blood to distant sites like lungs, liver, brain, spinal cord, and adrenals.
LEUKEMIAS
Leukemias are malignant neoplasms of the hematopoietic stem cells characterized by diffuse
replacement of the bone marrow by neoplastic cells.
General characteristic
In the major, the leukemic cells spill over into the blood, where they may be seen in large
number. These cells may also infiltrate the liver, spleen, lymph nodes and other tissues
throughout the body.
Traditionally, leukemias are classified on the basis of the cell type involved and the state of
maturity of the leukemic cells. Thus, acute leukemias are characterized by the presence of
very immature cells (called blasts) and by a rapidly fatal course in untreated patients.
On the other hand, chronic leukemias are associated, at least initially, with welldifferentiated (mature) leukocytes and with a relatively indolent course.
The type of acute and chronic leukemia is established on the basis of cytochemical
peculiarities of tumor cells.
Acute leukemias, despite differences in their cell of origin, share important morphologic
and clinical features. They are associated with replacement of normal marrow elements by a
sea of proliferating “blast cells” that do not seem to undergo normal maturation.
Classification of the leukemias
I. Acute leukemias:
1. Undifferentiated.
2. Myeloblast.
73
3. Lymphoblast.
4. Plasmoblast.
5. Monoblast.
6. Erythromyeloblast.
7. Megacaryoblast.
II. Chronic leukemias:
1. Of myelocyte origin:
Chronic myeloid.
Chronic erythromyelosis.
Erythremia.
True polycytemia (Vaquez-Osler syndrome).
2. Of lymphocyte origin:
Chronic lympholeukemia.
Skin lymphomatosis (Sezary’s disease).
3. Paraproteinemic leukoses:
Myeloma.
Primary macroglobulinemia (Valdenstrem’s disease).
Heavy chain disease (Franklin’s disease).
4. Of monocyte origin:
Chronic monocyte leukemia.
Histiocytosis.
Classification based on the number of leukemic cells in 1 mcl of blood:
1. Leukemic (tens and hundreds thousand leukemia cells per 1 mcl).
2. Subleukemic (not more that 15.00%-25.000 per 1 mcl).
3. Leukopenic (leukocyte count is reduced but leukemia cells can be found).
4. Aleukemic (leukemic cells in the blood are almost absent).
Morphology of all leukemias
There are two aspects to the morphologic features of leukemia:
1. The specific cytologic details of the leukemic cells seen in periferal blood smears and
bone marrow aspirates.
2. The tissue changes produced by infiltrations of leukemic cells.
The tissue alterations produced by various leukemias are often similar and may be
separated into primary changes, attributed directly to the abnormal overgrowth or
accumulation of white cells; and secondary changes, caused both by the destructive effects
of masses of these cells and by their relative infectiveness in protecting against infection.
Although leukemic cells may infiltrate any tissue or organ of the body.
Leukemic infiltration of hemopoietic organs (bone marrow, spleen, lymph nodes) at first,
then of the other organs (mucous membranes, myocardium, kidneys, brain, vessels, etc.).
The most striking changes are seen in the bone marrow, spleen, and liver: massive
splenomegaly, lymhp node enlargement, enlargement of the liver.
Pyoid bone marrow due to proliferation of the tumor cells (mature or immature,
respectively) in the bone marrow with displacement of the red sprout. Macroscopically,
bone marrow is grayish-whitish.
Necrotic tonsillitis, gingivitis develops due to leukemia infiltration of the oral mucosa and
tonsils against a background of immunogenesis inhibition. Besides, infiltrates in the gingiva
are particularly characteristic of monocytic leukemia.
The secondary changes of all forms of leukemia are:
a) Anemia and thrombocytopenia, especially in acute leukemia.
b) The bleeding diathesis.
c) Petechial and ecchymoses.
d) Hemorrhages into the serosal linings of the body cavities, mucosal
hemorrhages etc.
e) Disseminating intravascular coagulation may also lead to widespread bleeding.
f) And, finally, infections and sepsis are a prominent feature, especially in acute
leukemias.
Foci of extramedullary hemopoiesis develop in the liver, spleen, kidneys, and lymph nodes.
There is compensatory adaptation reaction directed to restoration of the red sprout.
74
Distinctive features of acute and chronic leukemia are:
1) Bone marrow and blood picture (in acute leukemia blasts are observed, in chronic mature
cells are found).
2) Leukemic failure (hiatus leukemicus) characterizes acute leukemia. It is sharp increase of
blast count and single mature elements while transitional forms are absent.
3) Sharp enlargement of the spleen, liver, kidneys and lymph nodes characterizes chronic
leukemia while in chronic one it is less marked. The spleen can weigh 6 - 8 kg, the liver 5 - 6 kg.
Complications and causes of death:
1) Hemorrhage to vital organs (brain).
2) Ulcerative necrotic and septic complications (sepsis).
Chronic myeloid leukemia (CML)
Chronic myeloid (myelogenous, granulocytic) leukemia comprises about 20% of all leukemias
and its peak incidence is seen in 3rd and 4th decades of life. A distinctive variant of CML seen in
children less than 3 years of age is called juvenile CML.
Clinical Features
At research of chromosome clearly recognize Philadelphia chromosome, associated with poor
prognosis.
Leukemic infiltration contains myelocytes and metamyelocytes.
Pyoid bone marrow.
Features of anemia such as weakness, pallor, dyspnoe and tachycardia.
Symptoms due to hypermetabolism such as weight loss, lassitude, anorexia, night sweats.
Splenomegaly and hepatomegaly is almost always present and is frequently massive.
Bleeding tendencies in blast crises.
Less commonly, features such as gout, visual disturbance, neurologic manifestations and
priapism are present.
Juvenile CML is more often associated with lymph node enlargement than splenomegaly.
Chronic lymphocytic leukemia (CLL)
Chronic lymphocytic leukemia constitutes about 25% of all leukemias and is
predominantly a disease of the elderly (over 50 years of age) with a male preponderance (male-female
ratio 2:1).
Clinical Features:
Features of autoimmune hemolytic anemia such as weakness, fatigue and dyspnoe.
Enlargement of superficial lymph nodes.
Splenomegaly and hepatomegaly are usual.
Hemorrhagic manifestations are found in CLL with thrombocytopenia.
Infections, particularly of respiratory tract, are common.
Paraproteinemic leukemias
The most important of this group is myeloma.
The disease is characterized by growth of tumor lymphoplasmocytic cells (myelomic cells)
in the bone marrow and other organs.
Bone marrow myelomatosis causes bone destruction. Sinusal resorption of the bone results
in osteolysis and osteoporosis. The bones become fragile.
Hypercalcemia develops due to their destruction; it may be followed by development of
calcific metastases.
Myelomic-cell infiltration develops in the inner organs: spleen, lymphatic nodes, liver,
kidneys, lungs, etc.
A number of changes are associated with secretion of paraprotein by the tumor cells. These
changes are amyloidosis, paraproteinemic nephrosis or myelomic nephropathy resulting in
shrunken kidney.
Depending on the character of myelomic cells, myelomas are divided into:
1. Plasmocyte.
2. Plasmoblast.
3. Polymorphocellular.
4. Small-cell.
75
Morphologically, depending on the character of myelomic infiltrations the following forms
are distinguished:
1. Diffuse.
2. Diffuse nodular.
3. Multiple nodular.
Causes of death are uremia, sepsis, necrotic changes, and amyloidosis.
LYMPHOMAS
Lymphomas are malignant neoplasms characterized by the proliferation of cells native to
the lymphoid tissues that is lymphocytes, histiocytes and their precursors and derivatives.
Like other neoplasms, all lymphomas are of monoclonal origin.
Two distinct clinicopathologic groups are distinguished:
I. Hodgkin’s lymphoma or Hodgkin’s disease (HD).
II. Non-Hodgkin ‘s lymphomas (NHL).
Non – Hodgkin’s lymphomas
The usual presentation of Non-Hodgkin’s lymphomas (NHL) is a localized or generalized
lymphadenopathy.
However, in about one-third of cases it may be primary in other sites where lymphoid tissue
is found, for example, in the oropharyngeal region, bone marrow, gut and skin.
All forms of lymphoma have the potential to spread from their origin in a single node or
chain of nodes to the other nodes, and eventually to disseminate to the spleen, liver and the
bone marrow.
There are some important principles of classification of NHL.
As all tumors of the immune system, NHLs may originate in T-cells, B-cells, or histiocytes.
The vast majority of NHL is of B-cells origin; the remainder is in large part of T-cells
tumors.
Tumors of histiocytes or macrophages are quite uncommon.
Histologically, the lymphoma cells exhibit two different growth patterns: they are either
clustered into identifiable nodules (nodular lymphoma) or spread diffusely throughout the
node (diffuse lymphoma). In general, nodular (or follicular) architecture is associated with
a significantly superior prognosis to that of diffuse pattern.
It may be recalled that normal B cells form follicles within lymph nodes; malignant B cells
tend to recapitulate this behavior with nodule formation. Not surprisingly, therefore,
nodular lymphomas are composed exclusively of B cells.
There are some categories of NHL. Every of this category includes some subtypes of
leukemias with their own morphological features:
1. Low-grade.
2. Intermediate-grade.
3. High – grade.
Low-Grade Lymphomas
This category includes three tumors: small lymphocytic lymphoma; follicular, predominantly
small cleaved cell lymphoma; and follicular, mixed (small cleaved and large cell) lymphoma.
Small Lymphocytic Lymphoma (SLL)
This pattern makes up approximately 4% of all NHLs and is the only low-grade lymphoma
that does not have a follicular architecture.
Microscopically: SLL consists of compact, small, apparently unstimulated lymphocytes with
dark-staining round nuclei, scanty cytoplasm, and little variation in size. Mitotic figures are
rare, and there is little or no cytologic atypia.
Involvement, of bone marrow to present in almost all cases, and in about 60% of patients
the neoplastic cells spill over into blood, evoking a chronic lymphocytic leukemia-like
picture.
Follicular Lymphomas
There are two cytologic subgroups of low-grade follicular lymphomas: follicular small
cleaved cell and follicular mixed cell type.
76
The neoplastic B cells tend to recapitulate normal lymphoid follicles, and hence they
resemble the cells seen within normal germinal centers.
Small-cleaved cells are slightly larger than normal lymphocytes, with scanty cytoplasm. The
most distinctive feature that differentiates the tumor cells from small normal lymphocytes
is their irregular “cleaved” nuclear contour, characterized by prominent clefts, indentations,
and linear enfolding.
The nuclear chromatin is coarse and condensed, and nucleoli are indistinct. Mitoses are
infrequent.
Follicular, mixed lymphomas constitute a small proportion of all follicular center cell
tumors.
Intermediate-Grade Lymphomas
There are four tumors in this category - one with a follicular architecture and the other three
with a diffuse pattern. The diffuse intermediate-grade lymphomas are distinguished on the basis of
their cellular composition.
Follicular, Predominantly Large Cell Lymphoma.
In contrast to the low-grade follicular lymphomas, the majority of the neoplastic cells are
large, with cleaved or noncleaved nuclei. Mitotic figures are also more numerous.
Diffuse Small Cleaved Cell Lymphoma.
This type is composed of small-cleaved cells that are morphologically and phenotypically
similar to those that are present in the follicular small-cleaved cell lymphoma.
Diffuse Mixed Small and Large Cell Lymphoma.
These tumors contain a mixture of small cleaved cells already described and large cells that
may be cleaved or noncleaved.
The nuclei of large cleaved cells are irregular in contour, indented, and larger than nuclei of
normal histiocytes or endothelial cells (often used as a reference in evaluating size).
The nuclear chromatin is slightly more dispersed than in a normal small lymphocyte, and
nucleoli are inconspicuous.
The cytoplasm is scant and pale.
Large noncleaved cells are up to four times the size of normal lymphocytes, with a round or
oval nucleus and one to two prominent nucleoli; the nuclear chromatin-is vesicular and
mitoses are prominent. The amount of cytoplasm is greater than in large cleaved cells and
stains pale blue.
Diffuse Large Cell Lymphoma.
This variant is the most common of intermediate-grade lymphomas.
Morphologically, these tumors contain predominantly large cells of the cleaved and
noncleaved types described above.
It should be noted that the distinction between diffuse large cell lymphomas and the diffuse
mixed variant is difficult and somewhat arbitrary.
High-Grade Lymphomas
There are 3 types of lymphomas in this category: (1) large cell immunoblastic lymphomas; (2)
lymphobtastic lymphoma, a tumor that occurs in adolescents and is associated with a characteristic
clinical presentation; and (3) small noncleaved lymphomas, which include Burkitt's lymphoma and
related B-cell neoplasms.
Large Cell Immunoblastic Lymphoma.
In some cases the tumor cells have plasmacytoid features.
These cells are four to live times larger than small lymphocytes and have a round or oval
large nucleus that appears vesicular owing to margination of chromatin at the nuclear
membrane. One or two centrally placed prominent nucleoli are usually seen.
In other cases, the turner cells may contain large multilobated (polymorphous) nuclei, or
the nucleus may be round with clear cytoplasm.
77
Although features such as plasmacytoid appearance and clear cytoplasm or polymorphous
nucleus is suggestive of B and T immunoblasts. Respectively, these distinctions are not
absolute.
Lymphoblastic Lymphoma
The tumors are fairly uniform in size, with scanty cytoplasm and nuclei that are somewhat
larger than those of small lymphocytes.
The nuclear chromatin is delicate and finely stippled, and nucleoli are either absent or
inconspicuous.
In keeping with its aggressive growth, the tumor shows a high rate of mitosis, and as with
other tumors having a high mitotic rate (e.g., Burkitt's lymphomas), a "starry sky" pattern
is produced by the interspersed benign macrophages.
Small Noncleaved Cell Lymphoma.
These tumors consist of a sea of strikingly monotonous cells, with round or oval nuclei
containing two to five prominent nucleoli.
The nuclear size approximates that of benign macrophages within the tumor. There is a
moderate amount of faintly basophilic cytoplasm, which also is intensely pyroninophilic
and often contains small, lipid-filled vacuoles (better appreciated on stained imprints of the
tumor).
A high mitotic index is very characteristic, as is cell death, accounting for the presence of
numerous tissue macrophages with ingested nuclear debris. Since these benign,
macrophages, which are diffusely distributed among the tumor cells are often surrounded
by a clear space, they create a “starry sky” pattern.
Hodgkin’s Disease or Lymphogranulomatosis
Hodgkin’s disease (HD) is a disorder involving primarily the lymphoid tissue. It arises
almost invariably in a single node or chain of nodes and spread characteristically to the
anatomically contiguous nodes.
It’s separated from NHL for several reasons.
1. First it is characterized morphologically by the presence of distinctive neoplastic
giant cells called Reed-Sternberg’s cells, admixed with a variable inflammatory
infiltrate.
2. Second, it is often associated with somewhat distinctive clinical features, including
systemic manifestations such as fever. Finally, the target cell of neoplastic
transformation has yet to be identified with certainty.
Morphology
A distinctive tumor giant cells known as the Reed-Sternberg cell (RS) is considered to be
the essential neoplastic element in all forms of HD, and their identification is essential for
the histologic diagnosis.
Classically it is a large cell, most often benucleate or belobed, with two halves often
appearing as mirror images of each other.
At other times there are multiple nuclei, or the single nucleus is multilobade and polypoid.
The nucleus is enclosed within the abundant amphophilic cytoplasm. Prominent within the
nuclei are large, inclusion-like, “owl-eyed” nucleoli generally surrounded by a clear halo.
Variants of RS cells include uninucleated cells with prominent nucleoli, and lacunar cells.
The lacunar cell is large with single hyperlobated nucleus containing multiple small nucleoli
and an abundant, pale-staining cytoplasm.
The origin of HD is unknown. The accumulated phenotypic and molecular studies suggest
that HD is heterogeneous with respect to both the cell type involved and the etiologic
agents. The nodular form of lymphocyte predominance type is clearly B-cell neoplasm;
others may arise from B-cells or T-cells.
There are some subtypes of HD according to the Rye classification:
1. Lymphocytic predominance HD. Characterized by a diffuse or vaguely nodular
infiltrate of mature lymphocytes admixed with variable numbers of benign histiocytes.
Scatterd among these cells are the distinctive RS cells. Most patients are under 35 years
of age and have an excellent prognosis.
78
2. Mixed cellularity HD is a common form of HD. Typical RS cells are plentiful. Usually
there is heterogenous cellular infiltrate, which includes eosinophils, plasma cells, and
histiocytes.
3. Lymphocyte depletion HD. This uncommon pattern is characterized by a paucity of
lymphocytes, RS and their pleomorphic variants, and also massive foci of necrosis and
sclerosis. The reticular variant is cellular and contains highly anaplastic, atypical RS
cells. Patients are usually older and have a very poor prognosis.
4. Nodular sclerosis HD. In this variant, fine or dense collagenous bands subdivide the
lymphoid tissue into circumscribed nodules. There are varying proportions of lacunar
cells and lymphocytes; classic RS cells are rare. Most patients are young with an
excellent prognosis
The causes of death and complications
1. Renal amyloidosis followed by shrunken kidney and uremia.
2. Intoxication.
3. Septic complications.
79
PART II. SYSTEMIC PATHOLOGY
DISEASES OF BLOOD
ANEMIAS
Anemia literary means “without blood, bloodless”, but indeed this term denotes a
complicated symptom-complex which is characterized by changes in the number of erythrocytes and
reduction of hemoglobin amount in a unit of blood volume.
Anemia is defined as a hemoglobin concentration in blood below the lower limit of the normal
range for the age and sex of the individual. In adults, the lower extreme of the normal hemoglobin is
taken as 13.0 g/dl for males and 11.5 g/dl for females.
It known that erythrocytes and hemoglobin realize transport oxygen to the tissues. Thus,
decrease in the number of erythrocytes may cause oxygen deficiency in the tissues, i.e. hypoxia
development.
Not only the degree of anemia but also the rate of its development as well as the degree and
quickness of the organism adaptation are important. Physicians often observe discrepancy between
the severity of anemia and active condition of the patient, which can be explained by compensation
mechanisms, providing physiological need of the tissues in oxygen. Only in cases of severe anemia or
at high rate of adaptation, hypoxia may develop.
Numerous neurohumoral factors participate in compensation of anemic state. They stimulate
blood and hemopoietic systems. Hypoxia causes appearance of incompletely oxygenated metabolic
products, which affect central regulation of blood system as well as neuromuscular apparatus of the
heart causing increase in the heart rate and acceleration of the blood flow. As a result, minute blood
volume discharged by the left ventricle increases twice (to 8 liters instead of 4). Besides, spasm of
peripheral vessels develops in anemia and blood reserves from the tissue depot (mainly from
subcutaneous tissue) enter the blood circulation.
Classification of anemias is based on the mechanism of production:
I. Anemia by blood loss (post hemorrhagic).
II. Anemia by impaired red cell production (deficient).
III. Anemia by increased rate of destruction of red blood cells (RBC) - hemolytic
anemia.
Morphologically, anemias can be classified on the basis of the size and shape of RBC in
peripheral smears and their content of Hb. There are three types of anemias, such as (table 7).
TABLE 7. Classification of anemias
1
Classification
Normocytic-normochromic anemia
2
Microcytic-hypochromic anemia
3
Macrocytic-normochromic anemia
Examples
Aplastic anemia
Posthemorrhagic anemia
Hemolytic anemia
Anemia of chronic disease
Iron-deficiency anemia
Sideroblastic anemia
Talassemia
Perniciosus anemia (lack of vit B12)
Folic acid deficiency
Blood mass in anemia may be normal, increased or decreased. These conditions are called
Normovolemia.
Hypervolemia.
Hypovolemia.
I. The anemia by blood loss (Posthemorrhagic anemias)
The anemia by blood loss (Posthemorrhagic anemias) is caused by the blood loss in
traumas, pathological processes, accompanied by damage of the vessel or hemorrhage from the inner
organs. Depending on the size of the injured vessel and the rate of the blood loss it may be acute or
chronic.
Acute posthemorrhagic anemia
80
Examples:
Massive hemorrhage from the vessels of the stomach and intestine in ulcer of the stomach
and duodenum.
From the ulcers in typhoid fever.
In ectopic (tubal) pregnancy.
Pulmonary hemorrhage in tuberculosis.
Rupture of aortic aneurysm.
Rupture of the heart walls due to transmural infarction.
In rupture of the aortic arch, loss of less than 1 liter of blood causes death due to sudden drop
in arterial pressure. The death occurs before exsanguinations of the organism; therefore anemia in the
organs is not marked. In hemorrhages from small vessels, death occurs when half of the blood is lost.
Morphological signs of hemorrhage:
Pallor of skin and internal organs, collapse signs.
In case of the fatal hemorrhage, the smallest hemorrhages (petechia) occur under
endocardium of the left ventricle (Minakov’s streaks).
If the hemorrhage is not fatal, the blood loss is compensated due to regeneration processes,
taking place in the tissue of the bone marrow. The bone marrow of the flat bones
proliferates and becomes bright. The yellow bone marrow replaced by red (hemopoetic)
one.
In repeated hemorrhages, extramedullary hemopoesis may take place in the spleen, liver,
lymphatic nodes and other organs.
The prognosis of the hemorrhagic anemia depends on the rate of blood flow:
Rapid blood loss of 1/4 of the total blood volume may cause shock.
Loss of 1/2 of the total blood volume is incompatible with the life.
Loss of 3/4 of the total circulating blood does not cause death if it occurs slowly during
several days.
In healthy persons, even at considerable blood loss, its composition restores in 4 - 5 weeks.
Chronic post hemorrhagic anemia
This frequently develops after long, repeated slow blood loss, in the majority of cases at
hemorrhages from gastrointestinal tract (ulcer, cancer, hemorrhoids), uterine bleedings, in
hemophilia, hemorrhagic diathesis, in ankylospondylosis.
A clinic-morphological signs of anemia are a pale skin and visceral organs. In some cases,
the source of hemorrhage is inconsiderable and very difficult to reveal. Severe irondeficiency anemia develops.
II. Anemias impaired red cell production (deficiency anemias)
The anemia appearing as a result of breach of hemopoesis is called by three ways:
a) deficient anemia,
b) impaired red cell production,
c) anemia of diminished erythropoesis.
Diminished erythropoiesis may be the result of deficiency of some vital substrate necessary for
red cell formation.
Included in this group are:
a)
Cytoplasmic maturation defects.
1. Deficient hem synthesis: Iron deficiency anemia.
2. Deficient globin synthesis: Thalassemic syndromes.
b)
Nuclear maturation defects.
1. Vitamin B12 and/or folic acid deficiency: Megaloblastic anemia.
c)
Defect in stem cell proliferation and differentiation.
1. Aplastic anemia.
2. Pure red cell aplasia:
Anemia of chronic disorders.
Bone marrow infiltration.
Congenital anemia.
Iron deficiency anemia
Iron obtained from diet showed replace its loss (about 1 mg daily) in an adult male or in a
non-menstruating female, while in a menstruating woman there is an additional iron loss of
0.5-1 mg daily.
81
The iron required for hemoglobin synthesis is derived from 2 primary sources - ingestion of
foods containing iron and recycling of iron from senescent red cells.
Iron is absorbed mainly in the duodenum and proximal jejunum. Iron from diet containing
hem is better absorbed than non-hem iron.
Absorption of non-hem iron is enhanced by factors such as ascorbic acid (vitamin C), citric
acid, amino acids, sugars, gastric secretions and hydrochloric acid.
Iron absorption is impaired by factors like medicinal antacids, milk, pancreatic secretions,
phytates and tannates contained in tea.
Non-hem iron is absorbed almost exclusively as ferrous form. Iron balance in the body is
maintained largely by regulating the absorptive intake by intestinal mucosal cells, so called
mucosal block.
Pathogenesis
It is only after the tissue stores of iron are exhausted that the supply of iron to the marrow
becomes insufficient for hemoglobin formation, iron deficiency anemia develops. One or more of the
following factors may cause it:
1. Increased blood loss.
Uterine e.g. excessive menstruation in reproductive years, repeated miscarriages, at onset
of menarche, postmenopausal uterine bleeding.
Gastrointestinal e.g. peptic ulcer, hemorrhoids, hookworm infestation, cancer of stomach
and large bowel, oesophageal varices, hiatus hernia, chronic aspirin ingestion, ulcerative
colitis, diverticulosis.
Renal tract e.g. hematuria, hemoglobinuria.
Nose e.g. repeated epistaxis.
Lungs e.g. hemoptysis.
2. Increased requirements.
Spurts of growth in infancy, childhood and adolescence.
Prematurity.
Pregnancy and lactation.
3. Inadequate dietary intake
Poor economic status.
Anorexia e.g. in pregnancy.
Eldery individuals due to poor dentition, apathy and financial constraints.
4. Decreased intestinal absorption.
Partial or total gastrectomy
Achlorhydria
Intestinal malabsorption such as in celiac disease.
Clinical features
Peripheral blood. Red cells are pale (hypochromic) and smaller than normal
(microcytic).
Marrow. Hyperplasia of normoblasts, associated with loss of sideroblasts and absence of
stainable iron in the reticuloendothelial cells.
Others organs. Alopecia, koilonychias, atrophies of the tongue and gastric mucosa.
Esophageal webs may appear, completing the Plummer-Vinson triad of hypochromic
microcytic anemia, atrophic glossitis, and esophageal webs. Chlorosis (called so because of
pale greenish color of skin in this disease).
Megaloblastic anemia
There are two principal types of megaloblastic anemia:
1. Pernicious anemia, the major form of vitB12 deficiency anemia.
2. Folic acid deficiency anemia.
VitB12 and folic acid are necessary factors of hemopoiesis. VitB12 enters the organism through
the intestinal tract. Absorbtion of vitB12 takes place in stomach, when it has the Castle’s intrinsic
factor. Additional cells of fundal glands of the stomach produce it. The connection of vitB 12 and
Castle’s intrinsic factor leads to formation of the complex of protein and vitamin. It is absorbed
through mucosa of stomach and ileum. Entrance of vitB12 and active folic acid to bone marrow
determines normal erythropoesis and activates maturation of red blood cells.
Pernicious anemia
82
If the additional cells of fundal glands don’t product gastromucoprotein the anemia is
called pernicious anemia (PA).
Addison and Birmer described it in 1868. It is catching hereditary. The additional cells are
injured. They are involuated prematurely.
The autoimmunity injury of additional cells takes place in this case too. There are
antibodies against the additional cells (gastric parietal cells).
As a result of deficiency of these cells the erythropoiesis is done in megaloblastic type.
The processes of destruction predominate on the processes of hemopoiesis in this case.
Megaloblastic and megalocytic disintegrate in bone marrow and in the focuses of
extramedullar hemopoesis and in the blood vessels too.
Morphology
There is general hemosiderosis as a result of disintegration of red blood cells.
There is a fatty change in the parenchymatous organs as a result of hypoxia grows.
The major specific changes in PA are found in bone marrow, alimentary tract and central
nervous system.
In the alimentary system abnormalities are regularly found in the tongue and stomach. The
tongue is shiny, glazed, and “beefy” (atrophic glossitis). The changes in the stomach are
following: the mucosa is thin, plain; its wrinkles are smooth away. The glands are
decreased. The epithelium is atrophied. These changes lead to sclerosis.
The liver increases in size, has a density consistence and a brown color (hemosiderosis).
Pancreas has a density consistence too (sclerosis).
The principal alterations involve the spinal cord, where there is myelin degeneration of the
dorsal and lateral tracts, sometimes followed by loss of axons. These changes give rise to
spastic paraparesis, sensory ataxia, and sever paresthesias in lower limbs.
Folic acid deficiency anemia
Folic acid deficiency induces a megaloblastic anemia that is clinically and hematologically
indistinguishable from that encountered in vitamin B12 deficiency. However, neurologic changes seen
in the latter do not occur and gastric atrophy is absent.
Hypoplastic and aplastic anemias
Characterized by failure or suppression of multipotent myeloid stem cells and resultant
neutropenia, anemia, and thrombocytopenia (pancytopenia). May be idiopathic or caused due to
following factors:
Endocrine (hypothyroidism, thymus tumors).
Radiation lesions (x-rays, radium radiation, atomic energy).
Chemical (benzene, cytostatic preparations, etc.
Toxicoallergic (drugs).
Infectious.
Destruction of the bone marrow by cancer metastases.
Morphology
Hypocellular marrow.
Hematopoetic cells replaced by fat cells.
Secondary effects of granulocytopenia (infections) and thrombocytopenia (bleeding).
III. Anemia by increased rate of destruction of red blood cells (RBC) - hemolytic anemia.
The process of destruction is predominated.
There are general hemosiderosis and hemolytic jaundice.
As a result of hemolysis of erythrocytes there is hyperplasia of bone marrow. It has a pinkred color.
There are many foci of extramedullar hemopoesis in lymphatic nodules, connective tissue
and spleen.
There are two groups of this type of anemia.
1. Hemolytic anemia with intravascular hemolysis predominates.
There are the following courses of this type such as hemolytic poisons, hard burns, malaria,
sepsis, and complications after hemotransfusions and transfusion of incompatible blood
groups. These anemias generate large amounts of free hemoglobin in circulation. Since this
83
free hemoglobin can’t be taken up fast enough by the phagocytic cells of the liver and
spleen, it is excreted through the glomeruli, causing hemoglobinuria.
Hemoglobin in the primary glomerular filtrate is partially taken up by the proximal tubular
cells and transformed into the hemosiderin inside the lysosome, causing renal
hemosiderosis.
Acute hemolytic anemia develops in poisoning with hemolytic poisons (mushrooms, venom
of snakes, phosphorus, etc.), in burns, sepsis, malaria, transfusion of incompatible blood,
fetal erythroblastosis. The latter occurs due to Rh incompatibility of the mother’s and
fetus’s blood.
2. Hemolytic anemia with extravascular hemolysis. Intrinsic defects (usually hereditary) are divided
into three groups, such as
1) Defects of cell membrane. This group includes hereditary spherocytosis,
elliptocytosis.
2) Defects of enzymes. Activity of enzymes decreases. And as a result of this
erythrocytes are destructured
3) Defects of molecular structure of hemoglobin (hemoglobinopathies). In some
hereditary disorders, the molecular structure of hemoglobin is abnormal.
It includes the following forms of the disease: congenital (family) spherical-cell
anemia, sickle-cell anemia, thalassemia, or Cooley's anemia.
Spherical-cell anemia is characterized by congenital spherocytosis (erythrocytes are small,
spherical, brightly colored, without light center, with decreased resistance). These abnormal
erythrocytes are destroyed. The first sign of the disease is jaundice; it is followed by splenomegaly and
anemia.
Sickle-cell anemia and thalassemia are hemoglobinopathies (conditions due to abnormal
hemoglobin in the erythrocytes). The cause of sickle-cell anemia is congenital insufficiency of
erythrocytes due to presence of S hemoglobin (S corresponds to sickle). The condition is characterized
by presence of sickle-like erythrocytes revealed during crisis; they cause stasis, hemorrhages, and
infarctions. Siderofibrosis caused by hemosiderin accumulation develops due to increased decay of
sickle-like erythrocytes in the spleen. :
Thalassemia (target cell anemia, Cooley’s anemia). It occurs in children and is characterized
by
Progressive anemia with erythroblastemia.
Enlargement of the spleen and liver.
Increased hemolysis.
Osteoporosis causing changes in the facial bones.
DISEASES OF CARDIOVASCULAR SYSTEM
ATHEROSCLEROSIS
Atherosclerosis is a multifactorial disease that affects the intima of elastic arteries. The
disease is characterized by intramural deposits of lipids, proliferation of vascular smooth
muscle cells and fibroblasts, and accumulation of macrophages.
Basic lesion is the patchy deposition of yellow lipid in plaques deep in the intima with
overlying fibrosis up to 1,5 cm in diameter, protruding into the vessels lumen. It is called
atheroma, i.e. it is essentially an intimal disease.
The term AS derives from the combination of athero - (‘porrige’), referring to the soft, lipidrich material in the center of a typical intimal plaque, and sclerosis (scarring),referring to
the connective tissue components.
The major clinical syndromes are related with ischemia, which is produced by narrowing of
the vascular lumen (coronary heart disease, peripheral vascular disease, cerebral
infarction), or from weakening of the arterial wall leading to aneurysm.
Atherosclerosis begins early in life and develops progressively over years, it is rarely
symptomatic in the first three decades, but thereafter the frequency of clinical
atherosclerotic events increases logarithmically. Because of its prevalence, as can be
considered epidemic in industrialized nations.
Every year approximately 1 million persons in the world experience either a myocardial
infarct or sudden cardiac death. Nearly all of them are the result of atherosclerotic coronary
disease.
84
Background etiological factors influencing the rich of or susceptibility to atheroma are
multiply and interrelated. The major background factors may be grouped into two main
categories:
I Endogenous (not modifiable)
1. Sex
Atherosclerotic coronary heart disease is predominantly a disease of men. Especially in
younger ages; the prevalence in men in the fourth decade is three times that in women. Possible
explanations for the sex differences include levels of estrogenic hormones and higher levels of highdensity lipoprotein, which is known to be antiatherogenic, in premenopausal women then in men.
2. Genetic factors (Heredity)
Evidenced in cases with clearly defined abnormalities of lipid metabolism. Apparent genetic
roles in familial predisposition to AS may be related to genetic effects on other risk factors, especially
hyperlipoproteinemia, hypertension and diabetes mellitus.
II Environmental (modifiable)
1. Diet.
Many studies have demonstrated the specific effects of diet on lipid and lipoprotein levels,
including the amount of dietary cholesterol ingested, the total number of calories from carbohydrates,
protein and fat, and the intake of alcohol and concentrated sweets (anti-oxidants including red wine
reduce the risk).
2. Metabolic diseases.
There are diabetes mellitus, myxedema, nephrosis, xanthomatosis, familial hyper
cholesteronemia.
Hypertension.
Cigarette smoking.
The component of cigarette smoking responsible for the acceleration of atherosclerotic events
is not known. It may be related to effects of the cigarette smoking on thrombosis or to increased
concentration of carboxygemoglobin in the blood of smokers.
3. Lack of physical exercise.
4. Other risk factors.
Other risk factors suggested being associated with AS obesity, physical activity, hyperglycemia,
stress, and coffee consumption.
These factors may act as increased blood lipids-cholesterol and lipoproteins. The risk is
correlated with elevated low-density lipoprotein (LDL), formed from the catabolism of very-lowdensity lipoprotein (VLDL) to a cholesterol ester-protein core that carries some 70% of the total
serum cholesterol. Atheroma is specifically associated with high blood low-density lipoprotein levels
(as well as total cholesterol levels). Risk is inversely related to the high-density lipoprotein (HDL)
levels, perhaps because HDL helps clear cholesterol from vessel lesion.
Pathogenesis of AS has three stages:
1. Endothelial injury is accompanied by the attachment of monocytes, platelets, and
thrombus formation.
2. Macrophages in the intima phagocytise lipid and transform into foam cells. Macrophages
also secrete growth factors that stimulate the proliferation of smooth muscle cells.
3. Ruptured atheromas release thrombogenic material into the circulation, causing thrombus
for intimal ulceration.
Classification AS has the following microscopically stages (phases):
1. Pre-lipid stage is characterized by mucoid swelling of intima and accumulation of plasma
proteins, and glycosaminoglycanes, the destruction of endothelium and elastic and collagen
fibers of intima's basal membrane.
2. Stage of fatty stripes (lipoidois). Fatty stripes appear on intima due to its lipid infiltration,
lipoproteins and proteins fixation. Lipids impregnate intima and are accumulated in
macrophages. Macrophages that have accumulated lipid in their cytoplasm appear
histologically as csantomic or foam cells. Elastic membranes become swollen, their
destruction occurs,
3. Stage of liposclerosis. Macrophages secrete growth factors and cytokines, which recruit
additional monocytes, macrophages and other cells. Cytokines and growth factors also
85
stimulate the proliferation of smooth muscle cells and their ingrowth into the intima from the
tunica media. Lipid accumulates not only in macrophages but also in smooth muscle cells.
From dead and dying cells, cholesterol is released into interstitial spaces. In the areas of
lipidosis a young connective tissue grows and forms a fibrous cap. On the luminal side,
atheromas typically covered with an intimal fibrous cap, consisting of fibroblasts, surrounded
by collagen, which replaces the normal intimal cells. Macroscopically dense, oval, white
formations are observed there.
4. Stage of atheromatosis is characterized by necrosis of the central part of fibrous cap with
forming of amorphous substance (atheromatouse detritis). Atheromas consist of amorphous
lipid-rich material and are soft. Cholesterol clefts are recognized by their typical needleshaped appearance.
5. Stage of ulceration is characterized by the break of the fibrous cap cover and forming of ulcer
with small hemorrhage into plaque.
6. Stage of atherocalcinosis is characterized by deposition of calcium in ulcerative plaque.
Dense and fragile cap is formed due to the cap of connective tissue infiltration with calcium.
The calcification of vessels leads to hardening of arteries. Atheromas weaken the arteries and
predispose to formation of aneurysm.
Complicated plaques develop from preexisting fibrous plaques as a result of one of a
combination of several pathologic changes that include calcification; ulceration, thrombosis and
hemorrhage. The complicated lesion is the most common type of atherosclerotic lesion that produces
significant circulatory change and clinical disease.
Clinical-morphological appearances
1. Atherosclerosis of aorta - the most common form. Usually it is not complicated by the
thrombosis, thromboembolism and embolism to legs. Development of aortal aneurysm is possible.
2. Atherosclerosis of coronary arteries of heart lead to ischemic heart disease (IHD). May be
causes acute infarction.
3. Atherosclerosis of arteries of cerebrum. It’s possible the development of thrombosis. The
results are ischemic infarctions of brain, less often the haemorrhage in brain occurs. Dystrophy and
atrophy of the brain cortex may develop as result of the long-term ischemia. General chronic ischemia
of brain leads to senile dementia. Atherosclerosis of carotides leads to acute local ischemia and
cerebral softening (infarction).
4. Atherosclerosis of renal arteries leads to atrophy of parenchyma, or infarction. Outcome is
atherosclerotic nephro-cirrhosis.
5. Atherosclerosis of arteries of an intestine is complicated by the thrombosis, leading to the
gangrene.
6. Atherosclerosis of arteries of extremities, very often this process is located in femoral
arteries. The thrombosis with gangrene of leg is possible. Collateral circulation is usually good;
atheroma must be very severe before chronic ischemia with intermittent claudication/or gangrene
develops.
Aneurysms
These are localized abnormal dilatations of vascular wall. Most common (and significant) are
aortic aneurysms. Morbidity and mortality are secondary to
Rupture.
Impingement on adjacent structures.
Occlusion of proximate vessels by either extrinsic pressure or superimposed thrombosis.
Embolism from mural thrombosis.
Etiologies of aneurysms include atherosclerosis, cystic medial necrosis (the two most
common causes), syphilis, trauma, congenital defects, and infections (mycotic aneurysms).
Morphologically, aneurysms are classified as follows:
86
Berry aneurysm. Spherical dilatation due to congenital wall weakness, generally less
than 1.5 cm in diameter, typically in the circle of Willis.
Saccular aneurysm. Large spherical dilatation up to 20cm in diameter, often at least
partially filled with thrombus. The etiology is usually atherosclerosis.
Fusiform (cylindroid) aneurysm. Gradual lumen dilatation generating a spindleshaped lesion up to 20 cm in diameter, and to the full length of the aorta. AS is the most
common cause.
Dissecting aneurysm. Blood enters the arterial wall through a tear, usually in the aortic
arch, and dissects the layers-typically between the middle and outer thirds of the media.
HYPERTENSION
In medically advanced countries, hypertension is the most common serious chronic disease,
affecting about a half of the population over 50 years of age. Arterial hypertension is defined clinically
as borderline when it riches 140/90 mm Hg and hypertensive when 165/95 mm Hg.
There is elevation of systolic pressure alone, (systolic hypertension) or elevation, of both
systolic - and diastolic pressure (diastolic hypertension), both have an increased risk of serious
complications, but diastolic hypertension is more dangerous.
Hypertension is classified into two types:
1. In 90-95% of all cases of hypertension, no cause can been established – such cases are called
essential or idiopathic or primary.
2. In only 5-10% of all cases of hypertension is any disease, which may be associated with disturbance
of these mechanisms detectable – such cases are secondary hypertension. Examples:
Kidney diseases.
Hyperfunction of adrenal cortex (Cushing ‘s syndrome – corticosteroid excess).
Tumor of adrenal medulla (pheochromocytoma) – catecholamine excess.
Hypertension occurs in toxemia of pregnancy.
This hypertension comprises 5-10% causes of disease.
If these causes of secondary hypertension were eliminated, hypertension disease would be
cure.
According to the clinical course, both types of hypertension may be benign or malignant.
1. Benign hypertension is moderate elevation of blood pressure and the rise is slow as the years pass.
About 90% of patients of hypertension have benign disease.
2. Malignant hypertension is marked and rapid increase of blood pressure to 200/140 mm Hg or
more and the patients have papilledema, hemorrhages and hypertensive encephalopathy.
All the above mechanisms are essentially vaso-constrictor. The possible roles of vaso-dilator
mechanisms – for example the effect of nitric oxide on vascular smooth muscle – are being currently
researched.
The increased peripheral resistance resulting in sustained hypertension may arise from:
1. Increased sympathetic tone.
2. Increased release of renin and generation of angiotensin.
3. The presence of vasoconstrictive substances in the circulation.
4. Increased sodium load and extracellular fluid load, and finally.
5. A postulated excessive responsiveness to the other factors.
Morphology
It is important to realize that the central lesion in most cases of hypertension is a decrease in
the size of the lumen in small muscular arteries and arterioles, the resistance vessels that
control the flow of blood through the capillary bed.
The lumen may be restricted by active contraction of the vessel wall, an increase in the
structural mass of the vessel wall, or both.
The morphologic changes associated with moderate elevations of blood pressure are too
subtle to be detected by simple histological studies. Small muscular arteries show segmental
dilatation as a result of necrosis of smooth muscle cells.
The combination of cell necrosis and deposition of plasma proteins in the vessel wall is
termed fibrinoid necrosis.
The period of acute injury is rapidly followed by smooth muscle proliferation and a striking
increase in the number of layers of smooth muscle cells, which yields the so-called onionskin appearance. Taken together, these changes are labeled malignant arteriosclerosis or
malignant arteriolosclerosis, depending on the size of the vessels affected.
87
Clinical-morphological stages
1. Subclinical stage occurs by hypertrophy of muscular layer and elastic structures of arterioles
and small-sized arteries, spasm of arterioles. At this stage the hypertrophy of the left ventricle
of heart begins.
2. A stage of general changes of arteries begins as arterial pressure increases. Arteriolar walls
permeability is increased, it results in plasmatic impregnation and hyalinosis. Elastic,
muscular-elastic and muscular arteries walls undergo elastofibrosis and atherosclerosis.
Elastofibrosis is characterized by a hyperplasia and breaking of internal elastic membrane and
spreading of connective tissue. Atherosclerotic changes in case of hypertension are more
extensive, the process reaches small-sized arteries of muscular type, plaques are more often
circular, that cause acute mechanical stenosis of the vessel.
3. The stage of secondary changes of organs is developed in connection with changes of arteries
and insufficiency of the intraorganic blood circulation. These changes develop slowly, that
results in atrophy of parenchyma and sclerosis (it’s characteristic of benign hypertension),
quickly (spasm, thrombosis, fibrinoid necrosis) and causing infarctions and hemorrhages ( it’s
characteristic of malignant hypertension).
The main clinical-morphological forms of essential hypertension
1. Cardiac form
Hypertensive heart disease or hypertensive cardiomyopathy is the disease of the heart
resulting from systemic hypertension of prolonged duration and manifesting by left
ventricular hypertrophy.
Often hypertension predisposes to atherosclerosis. The arterial changes and vascular
complications increase with the severity and duration of the hypertension, but are modified
by genetic factors, environmental factors, sex (females tolerate hypertension better), and
associated diseases.
Macroscopically, the most significant finding is marked hypertrophy of the heart, especially
of the left ventricle. Weight of heart reaches 1 kg, thickness of left ventricle walls is up to 3
cm, the papillary muscles are rounded and prominent, and the cardiac chamber is small
(concentric hypertrophy). But when decompensation and cardiac failure develop, there
is eccentric hypertrophy (myogenic dilation) with thinning of the ventricular wall
and dilation of the left ventricular and atrial cavities.
There may be dilatation and hypertrophy of right heart as well. Heart is called “cor
bovinum”.
2. Cerebral form (Cerebrovascular diseases.
It is characterized first of all as impairment of cerebral blood circulation. This hypertension
can result in two main types of parenchymal diseases of the brain:
1) Ischemic brain damage (hypoxic encephalopathy and cerebral infarction).
The pathologic appearance of the brain in hypoxic encephalopathy varies depending on the
duration and severity of hypoxic episode and the length of survival.
Macroscopically, there is focal softening. The area supplied by distal branches of the
cerebral arteries suffers from the most severe ischemic damage and may develop border
zone or watershed infarcts in the adjacent zones between the territories supplied by major
arteries.
Microscopically, the nerve cells die and disappear and are replaced by reactive fibrillary
glia.
Cerebral infarction is a localized area of tissue necrosis caused by local vascular occlusion.
Cerebral infarction may be anemic or hemorrhagic.
Macroscopically, an anemic infarct becomes evident 6 - 12 hours after its occurrence. The
affected area is soft and swollen and there is blurry of junction between gray and white
matter. Within 2-3 days, the infarct undergoes softening and disintegration.
A hemorrhagic infarct is red and superficially resembles a hematoma. It is usually the result
of fragmentation of occlusive arterial emboli or venous thrombosis.
2) Intracranial hemorrhage (intracerebral and subarachnoid hemorrhage).
88
Hemorrhage into the brain of patient with hypertension is intracerebral hemorrhage, which
is usually of hypertensive origin due to rupture of microaneurysm.
The common sites of hypertensive intracerebral hemorrhage are the region of the basal
ganglia, medulla and cerebellum cortex.
About 40% of patients die during the first 3-4 days of hemorrhage, mostly from hemorrhage
into the ventricles.
The outcome of intracerebral hemorrhage is cyst formation. Patients can be paralyzed.
3. Renal form
Renal form is characterized by chronic arteriolo-sclerotic nephrosclerosis.
Kidneys have a term “primary shrunken kidneys”.
Macroscopically, both kidneys are affected equally and are reduced in size and weight, often
weighting about 6 gm. The capsule is connected densely to the cortical surface. The surface
of the kidney is finely granular and shows V-shaped areas of scarring. The cut surface shows
firm kidney and narrowed cortex.
Microscopically, there are primary diffuse vascular changes, which produce parenchymal
changes and secondary as a result of ischemia. There is variable degree of atrophy of
parenchyma; these include glomerular shrinkage, deposition of collagen in Bowman's
space, periglomerular fibrosis.
Clinical features are variable, elevation of the blood pressure with headache, dizziness, and
palpitation.
Renal failure and uremia may occur.
In case of malignant hypertension can develop as hypertonic crisis - acute increase of
arterial pressure in communication (connection) with spasm of arterioles.
Morphological appearance of hypertonic crisis: plasmatic impregnation or fibrinoid
necrosis of arteriolar walls.
The causes of death among hypertensive patients are the following:
Congestive heart failure.
Coronary artery disease.
Cerebrovascular accidents.
Uremia.
Causes unrelated to hypertension. The cardiac complications therefore account for 36% of
the death.
ISCHEMIC HEART DISEASE
Ischemic heart disease (IHD) is the generic designation for a group of closely related
syndromes resulting from ischemia – an imbalance between the supply and demand of the heart for
oxygenated blood. Ischemia comprises not only insufficiency of oxygen (hypoxia, anoxia) but also
reduced availability of nutrient substrates and inadequate removal of metabolites. Because coronary
artery narrowing or obstruction owing to atherosclerosis underlies myocardial ischemia in the vast
majority of cases, IHD is often termed coronary artery disease (CAD) or coronary heart disease
(CHD).
The etiology and pathogenesis
Etiology of IHD is identical to the one of atherosclerosis and hypertension. Direct reasons of
development of the myocardial infarctions are spasms of vessels, thrombosis or thromboembolism of
coronary arteries of heart. Pathogenic factors (factors of risk) are:
1. Hyperlipidemia;
2. Arterial hypertension;
3. Steatosis;
4. Hypodynamia;
5. Smoking;
6. Impairments of tolerance to carbohydrates;
7. Diathesis;
8. Genetic predisposition;
9. Sex-binded.
89
Depending on the rate of development and ultimate of the arterial narrowing and the
myocardial response, four ischemic syndromes may result:
1. Angina pectoris.
2. Myocardial infarction.
3. Sudden cardiac death.
4. Chronic ischemic heart disease.
Acute Ischemic Heart Disease (AIHD)
Angina pectoris
It is a symptom complex of IHD characterized by paroxysmal attacks of substernal or
pericordial chest discomfort (variously described as constricting, squeezing, choking, or knife-like)
caused by transient (15 sec. to 15 min.) myocardial ischemia that falls short of inducing the cellular
necrosis that defines infarction.
There are three somewhat distinctive patterns of angina pectoris, differentiated on the basis of
the provocation and severity of the pain:
1. Stable (typical) angina pectoris appears to be reduction of coronary perfusion to a critical
level by chronic stenosing coronary atherosclerosis; this renders the heart vulnerable to
further ischemia whenever there is increased demand, such as that produced by physical
activity, emotional excitement, or any other cause of increased cardiac workload.
2. Prinzmetal’s variant refers to a pattern of episodic angina that occurs at rest and has been
documented to be due to coronary artery spasm.
3. Unstable angina refers to a pattern of pain that occurs with progressively increasing
frequency, is precipitated with progressively less effort, often occurs at rest, and tends to be of
prolonged duration. This syndrome is sometimes referred to as preinfarction or acute
coronary insufficiency. Unstable angina is induced by fissuring, ulceration, or rupture of an
atherosclerotic plaque with superimposed partial thrombosis and possibly embolization or
vasospasm.
Acute myocardial infarction (MI)
Acute myocardial infarction also known as “heart attack”, is overwhelmingly the most
important form of IHD in industrial nations.
Pathogenesis. At least 90% of transmural acute MI are caused by an occlusive intracoronary
thrombus overlying an ulcerated or fissured stenotic plaque. Occlusion of a major coronary artery
results in ischemia throughout the anatomic region supplied by that artery, most pronounced in the
subendocardium. The function becomes strikingly abnormal within 1 min after ischemia, but
myocardial coagulation necrosis occurs only after 20 to 40 min of severe ischemia.
Classification of Myocardial infarction
I. According to localization: left ventricle, right ventricle, and right atrium, left atrium.
Infarctions are most frequently located in the left ventricle. Besides it may be located in other
parts of heart, but this is observed rarely. The region of infarction depends upon the area of
obstructed blood supply by one or more of the three coronary arterial trunks:
1) Stenosis of the left anterior descending coronary artery is the most common (40-50%) - the
infarctions of the anterior wall of left ventricle near apex or anterior two-thirds of interventricle
septum.
2) Stenosis of the right coronary artery is the next most frequent (30-40%) - interior/posterior
wall of left ventricle; posterior one-third of interventricular septum, posterior right ventricular free
wall in some cases.
3) Stenosis of the left circumflex coronary artery is seen least frequently (15-20%) - lateral wall of
left ventricle.
II. According to the anatomic region of the left ventricle: anterior, posterior, lateral, septal and
circumferential.
III. According to the degree of thickness of the ventricular wall:
90
1. Full-thickness or transmural, in which the ischemic necrosis involves the full or nearly full
thickness of the ventrical wall in the distribution of a single coronary artery. As a result the
rupture of cardiac wall, endocarditis with thrombus and fibrinous pericarditis can develop.
2. Subendocardial or lamina constitutes an area of ischemic necrosis limited to the inner onethird or at most one-half of the ventricular walls, often extending laterally beyond the
perfusion territory of a single coronary artery.
3. Subepicardial is rare infarction. In region of it fibrinous inflammation of pericardium
develops. It is called reactive pericarditis.
IV. According to the duration of infarctions:
1. Acute myocardial infarction develops in the first time (during 8 weeks from beginning of
ischemic necrosis).
2. Repeated myocardial infarction develops after 8 weeks of acute infarction.
3. Recurring (recidivic) myocardial infarction develops during 8 weeks of acute infarction.
Morphology
The macroscopic and microscopic changes in the myocardial infarction correspond to the
age of the infarct.
In 6-12 hours the lesion may have a slight pallor but may be inapparent; however, changes
in as early as 3 to 6 hours may be accentuated by use histochemical techniques.
By 18-24 hours infracted tissue is pale to cyanotic.
During first week the lesion becomes progressively more sharply defined, the color of
infarction is charged from cyanotic red to bright yellow or yellow-green. The consistency of
infarct in this period is soft.
A circumferential rim of hyperemic granulation tissue that progressively expends may be
seen by 7 to10 days.
Fibrous scar is well established by 6 weeks. It is thin, gray-white, hard, shrunken fibrous
scar.
Microscopically, within one hour of ischemic injury, there is intercellular edema, and
myocytes become wavy and buckled. This is attributable to stretching of noncontractile dead fibers by
adjacent viable contracting myocytes. In addition, border-zone viable cells show fine lipid droplets
and large cytoplasmic vacuoles called vacuolar degeneration or myocytolysis. At this stage, typical
coagulative necrosis is not yet evident.
In 12 to 72 hours a neutrophilic infiltrate into necrotic tissue with progressive evolution of
characteristic eosinophilic coagulative necrosis can occurs.
Between 3 and 7 days dead myocytes begin to disintegrate and are resorbed by
macrophages and enzyme proteolysis.
At 7 to 10 days granulation tissue appears and progressively replaces necrotic tissue,
ultimately generating a dens fibrous scar.
In fourth to sixth week increased fibrous tissue, decreased blood supply, fewer pigmented
macrophages, lymphocytes and plasma cells are seen.
Complications of infarction
Complications of infarction depend on the size and location of the necrosis, as well as the
reserve of functional myocardium.
1. Arrhythmias are the most common form of complication in acute myocardial infarction (75 to
95%).
2. Left ventricular congestive failure and mild-to-severe pulmonary edema (60%).
3. Cardiogenic shock (10%).
4. During the first weak the heart rupture may develop, which is often fatal. Rupture of the free
wall causes pericardial hemorrhage and tamponade. Rupture of the septum produces a left-to
right shunt with right heart volume overload.
5. Fibrinous pericarditis appears on the second day of myocardial infarction.
6. About 3 - 4% of patients who suffered from acute myocardial infarction develop postmyocardial infarction syndrome, which is characterized by pneumonitis.
7. Mural thrombosis and thromboembolism from intracardiac thrombi and thrombosis in the leg
veins is observed in 15-45% cases of acute myocardial infarction.
91
8. Cardiac aneurysm often occurs in the left ventricle, it impairs the function of the heart and is
the site for mural thrombi.
9. Dressler’s syndrome. It is immunocomplexis reaction to decomposition’s products of the
necrotic tissue with formation pericarditis and right-side pleurisy.
The main causes of death in this case are complications
1.
2.
3.
4.
Cardiogenic shock.
Tamponade of heart.
Thromboembolism.
Acute cardiac insufficiency.
Sudden cardiac death (SCD)
Defined as unexpected death from cardiac causes early after or without the onset of
symptoms. In the vast majority of cases in adults SCD is a complication and often the first
clinical manifestation of IHD. Infrequently, it is a consequence of myocarditis, mitral valve
prolapse, or hypertrophic cardiomyopathy.
The ultimate mechanism of death is almost always a lethal arrhythmia, presumably
triggered by previous conduction system scarring, acute ischemic injury, or electrical
instability due to electrolyte imbalance.
Morphology. Marked coronary atherosclerosis with critical (greater than 75%) stenosis
involving more than one of the three major vessels is present in 80 to 90 % of victims; only
10 to 20% of cases are of nonatherosclerotic origin.
Chronic Ischemic Heart Disease (CIHD)
The designation of CIHD is used for the heart of patients often but not exclusively elderly,
who individuously develop congestive heart failure (CHF), sometimes fatal, as a
consequence of ischemic myocardial damage.
Most cases of CIHD constitute simply postinfarctional cardiac decompensation or slowly
ischemic myocyte degeneration.
Cardiosclerosis can be local postinfarctional and diffuse atherosclerotic.
Macroscopically: on section white foci of sclerosis or brown coloration of the heart,
hypertrophy of left ventricle or, in contrary, some reduction in heart size, moderate to
severe multivessel stenosing coronary atherosclerosis and sometimes total occlusions
resulting from organized thrombi.
Microscopically: diffuse or local perivascular and interstitial fibrosis. In some cases the
hypertrophy of adjacent myocytes occurs, in others cases myocytic atrophy with perinuclear
deposition of lipofuscin appears.
Due to postinfarctional cardiosclerosis the chronic heart’s aneurysm may develops.
The pericardial surface of the heart in CIHD may have adhesions as the result of healing of
pericarditis also associated with post myocardial infarctions.
Cardiosclerosis leads to chronic cardiac insufficiency, which is characterized by congestion:
edema, cyanosis, petechias; indurations of organs (lungs, kidneys, spleen) and development
of the “nutmeg” liver.
Patients may die due to cardiacdecompensation or thromboembolism.
Cardiac hypertrophy and dilation
The heart may undergo compensatory enlargement in the form of hypertrophy, dilation, or
both, so as to prevent or postpone heart failure.
Compensatory hypertrophy
Hypertrophy of the heart is defined as an increase in size and weight of the myocardium. It
generally results from increased pressure load while creased volume load (e.g. valvular
incompetence) results in hypertrophy with dilatation of the affected chamber due to
regurgitation of the blood through incompetent valve. The atria may also undergo
compensatory changes due lo increased workload.
It appears that stretching of myocardial fibers in response to stress induces the cells to
increase in length. The elongated fibers receive better nutrition and thus increase in size.
Causes:
1. Left ventricular hypertrophy. The common causes of left ventricular hypertrophy are:
a) Systemic hypertension.
92
b) Aortic stenosis and insufficiency.
c) Mitral insufficiency.
d) Coarctation of the aorta.
e) Occlusive coronary artery disease.
f) Congenital anomalies like septal defects and patent ductus arteriosus.
g) Conditions with increased cardiac output: thyrotoxicosis, anemia, and arteriovenous fistulae.
2. Right ventricular hypertrophy. Most of the causes of right ventricular hypertrophy are due to
pulmonary arterial hypertension. These are:
a) Pulmonary stenosis and insufficiency.
b) Tricuspid insufficiency.
c) Mitral stenosis and/or insufficiency.
d) Chronic lung diseases: chronic emphysema, bronchiectasis, pneumoconiosis, pulmonary
vascular diseases, etc.
e) Left ventricular hypertrophy.
Compensatory dilation. Quite often, hypertrophy of the heart is accompanied by cardiac
dilation.
Causes:
a) Valvular insufficiency (mitral and/or aortic insufficiency in left ventricular dilatation,
tricuspid and/or pulmonary insufficiency in right ventricular dilatation).
b) Conditions with high cardiac output e.g. thyrotoxicosis, arteriovenous shunt.
c) Myocardial diseases: cardiomyopathies, myocarditis.
d) Systemic hypertension.
Hypertrophy of the myocardium without dilatation is referred to as concentric, and when
associated with dilatation is called eccentric. The weight of the heart is increased above the
normal, often over 500 gm.
Macroscopically, the thickness of the left ventricular wall above 15 mm is indicative of
significant hypertrophy. In concentric hypertrophy, the lumen of the chamber is smaller
than usual, while in eccentric hypertrophy the lumen is dilated. In pure hypertrophy, the
papillary muscles and trabeculae cameae are rounded and enlarged, while in hypertrophy
with dilatation these are flattened.
Microscopically, there is increase in size of individual muscle fibres. There may be multiple
minute foci of degenerative changes and necrosis in the hypertrophied myocardium.
RHEUMATIC DISEASES
Rheumatic diseases are group of collagen or systemic connective tissue diseases including
rheumatic fever, rheumatoid arthritis, systemic lupus erythematosus, scleroderma,
dermatomyositis and polyarteriitis nodosa, and Bechterew’s disease.
They are characterized by affect of collagen or connective tissue due to disturbances of
immune homeostasis.
Disturbances of immune homeostasis, development of autoimmune reactions, formation of
the toxic immune complexes and sensibilizated cells, injury of microcirculation with
following systemic progressive disorganization of connective tissue are main links of
pathogenesis of rheumatic diseases.
General characteristic of rheumatic diseases
Presence of chronic infectious focus.
Presence of early systemic changes of microcirculation.
Presence of hypersensitivity of immediate type with development of exudative – necrotic
reactions and hypersensitivity of delayed type with formation cellular infiltration.
Systemic progressive disorganization of connective tissue includes mucoid swelling,
fibrinoid changes, cellular reactions, sclerosis. Combination of different phases of
connective tissue disorganization, which indicates the chronic character of the diseases.
Chronic recurrent diseases with alternation of periods of exacerbation and remission
Genetic and environmental factors are important for development of these diseases. Thus,
rheumatic arthritis has less severe course in the residents of Africa than in those of Europe,
Lupus erythematosus is more frequent in Europian countries and USA than in Great
Britain.
93
RHEUMATIC FEVER (RF) and RHEUMATIC HEART DISEASE (RHD)
RF is an acute, inflammatory, recurrent disease mainly of children (ages 5 to 15) that
typically occurs 1 to 5 weeks after a group A streptococcal infection (usually sore throat).
Acute RF occurs after the infection with beta-hemolytic streptococci group A. The various
manifestations of the disease in the heart and other regions of the body, excluding the
initial infection (tonsillitis, nasopharyngitis), are not the result of a direct infection.
Most evidence suggests is secondary to host antistreptococcal antibodies that are crossreactive to cardiac antigens, but microbe initiated autoimmune reactivity is not ruled out.
Rheumatic Fever is thus a disease that involves many regions of the body, but it is not
serious import to the patient unless it involves the heart. It has been said, “rheumatic fever
licks the joints but bits the heart”.
Death is rare during acute RF, being secondary usually to the myocarditis. Typically, the
myocarditis and arthritis are transient and largely resolve, but the valvular involement may
lead to deformed, scarred valves with permanent dysfunction (chronic RHD) and
subsequent CHF.
Chronic RHD is more likely to occur when the first attack is in early childhood, when the
first bout of RF is severe, or with recurrent attacks.
Diagnosis rests on the clinical history and the presence of five major (Jones) criteria:
1. Erythema marginatum. Seen in children more often than adults. There is specific skin
“rush”, typically in a bathing suit distribution, macular lesions with erythematous rims and
central clearing.
2. Sydenhams chorea. A neurologic disorder with rapid, involuntary, purposeless movements.
3. Carditis. It may be myo-, endo-, or pericarditis.
4. Subcutaneos nodules. Seen in children more often than adults. Histologically, giant Aschoff
bodies are noted.
5. Migratory large joint polyarthritis.
Minor criteria:
1.
2.
3.
4.
Fever.
Arthralgia.
Longer PR interval in the ECG.
Leukocytosis.
Also may develop:
Rheumatic glomerulonephritis, rheumatic pneumonia is visceral changes.
Hyperplasia of lymphatic tissue, marked plasmatization is observed in the immune system.
Rheumatic vasculitis with fibrinoid changes of the walls. In the capillaries, there is
endothelium proliferation followed by desquamation, so-called rheumatic endotheliosis.
Vascular permeability increases sharply. The disease results in vascular sclerosis
(arteriolosclerosis, arteriosclerosis, capillarosclerosis).
Pathogenesis and Morphology of RF
A widely accepted concept of the nature of RF is that it is one of the so-called immune
disorders of connective tissue, the principal lesions being in the connective tissues throughout the
body, especially in the heart. RF has four stages.
Mucoid swelling. In the early phase of development of the lesions, edema of the
connective tissues is associated with an increase of mucopolysaccharide. The collagen fibers
are pushed apart by the accumulating of basophilic ground substance, and subsequently
they undergo swelling, fraying, fragmentation, and disintegration.
Fibrinoid changes. The affected areas, including collagen fibers and the ground
substance, are altered considerably and take on a deeply eosinophilic appearance
resembling fibrin; thus, the change is referred to as fibrinoid degeneration or necrosis.
Cellular reactions. The early exudative and degenerative features are followed by
proliferation, that is, an infiltration by lymphocytes, plasma cells, histiocytes, and
fibroblasts. The most distinctive proliferative lesion is the granulematous phase of the
Aschoff body.
94
Sclerosis. Aschoff bodies or diffuse inflammatory cellular infiltration are slowly replaced
by fibrous scar mainly about the vessels.
Pathognomonic focal inflammatory nodules called Aschoff bodies are the most
characteristic in the heart, but similar lesions may occur elsewhere. Three phases or stages in the
development of the Aschoff body are recognized:
1. Early (exudative, degenerative, or alterative) phase. These constitute foci of fibrinoid necrosis,
initially surrounded by lymphocytes, macrophages and a few plasma cells. The early phase of
the life cycle of the Aschoff body occur up to the fourth week of acute RF.
2. Intermediate (proliferative or granulematous) phase. In the intermediate phase, which is
evident during the fourth to the thirteenth week of the disease, cellular proliferation is the
dominant feature. Distinctive plump histiocytes (Aschoff or Anitschkow cells), some of which
are multinucleated (Aschoff multinucleated giant cells with abundant basophilic cytoplasm),
appear in periphery of nodules. Anitschkow cells are mononuclear cells. They have a moderate
amount of faintly stained cytoplasm with vaguely defined borders. Their nuclei are large and
vesicular and contain a prominent central chromatin mass that longitudinal section is serrated
(caterpillar-like). In cross section a halo is observed about the chromatin bar so that the
nucleus has an “owl-eye” appearance.
3. Late (senescent, fibrous, healing, or healed) phase. In 3 to 4 months, the healing phase is
reached, characterized by regression and fibrosis of the nodule. The collagenous fibers fuse to
form dense collagenous bindles, resulting in small scars between the muscle bundles,
frequently perivascularly.
Clinical-anatomical forms of Rheumatic Fever:
1. Cardiovascular form occurs endocarditis, myocarditis, pericarditis.
2. Polyarthritic form occurs migratory large joint polyarthritis (knee, cubital, humeral, hip joint,
ankle-joint). It is characterized by serous or serous-fibrinous inflammation. In the synovial
membrane the mucoid swelling develops. Articular cartilage is safe, therefore deformation
and ankylosis is absent.
3. Nodular (nodules around vessels) form occurs deposition of giant Aschoff bodies under skin
and may develop perivascular sclerosis.
4. Cerebral form occurs chorea. The damage of the brain is connected with rheumatic
vasculitis. Nervous cells degeneration, brain destruction and hemorrhages occur in the brain.
If these changes are clearly marked, they may cause chorea minor (in children).
Cardiovascular form
The cardiac involvement in RF is that of a pancarditis; that is, there is endocarditis,
myocarditis, and pericarditis.
Endocarditis (valvulitis)
The most prominent changes develop in mitral and aortic valves. Lesions may also be
present on the chordae tendineae, particularly at their attachment to the leaflets, and are
rarely on the papillary muscles of the left ventricle.
According to A.I. Abrikosov, valvular endocarditis is classified as follows:
1. Diffuse or valvulitis. In the active acute stage of the disease the valve leaflets or cusps are
thickened and lose their transparency. Edema with swelling of the leaflet, an increased
number of capillaries, and an infiltration by lymphocytes and occasionally by neutrophils are
seen. Plasma cells and fibroblasts may be present. In some instances, this nonspecific
inflammatory reaction may be all that occurs. Usually, however, there is also an increase in
acid mucopolysaccharide, with alteration of collagen and the fibrinoid change near the surface
of the valve and with surface deposition of fibrin from the blood in the ventricular cavity.
2. Acute verrucous endocarditis. This is followed by the appearance of characteristic wartlike
nodules (verrucae) ranging from 1 to 3 mm in diameter, mainly along the line of closure of the
cusps. It may lead to thickening, shortening, and blunting of valvular leaflets. Microscopically:
fibrinoid necrosis with thrombotic masses.
95
3. Fibroblastic or healing of the rheumatic valvulitis. The following changes take place:
a) Fibroblastic proliferation and collagen formation throughout the valve with scarring,
thickening, and rigidity of the leaflets.
b) Organization of the vegetations, with greater thickening along the line of closure.
c) Adhesions between the lateral portions of the cusps, particularly in the region of the
commisures.
d) Thickening, shortening, and fusion of the chordae tendineae.
e) Frequently, calcification, with contributes to the rigidity of the valve.
4. Relapsing verrucous endocarditis.
The result is deformity of one or more valves, especially mitral or aortic.
In the chronic or recurrent condition, the functionally important lesions are those of the
valves, which result in heart failure because of the increased work of the heart caused by the
valvular stenosis of insufficiency.
Mitral insufficiency
The pathophysiology of mitral regurgitation is complex.
Proper closure of the mitral valve depends not only on the mitral valve leaflets by
themselves but also on several additional functional components of the mitral valve
apparatus, namely, the chordae tendineae, the papillary muscles, and the left ventricle.
Valvular insufficiency may result because of retraction of the scarred leaflets in the vertical
direction leading to shortening of the cusps.
Changing hemodynamic conditions may dramatically improve or worsen the degree of
mitral regurgitation.
Mitral insufficiency and stenosis are commonly combined.
When mitral insufficiency is the main alteration, the effects are the follows:
a) Dilatation and hypertrophy of the left ventricle.
b) Dilatation and hypertrophy of the left atrium, often greater than in mitral stenosis.
c) Effects on the right side of the heart as in mitral stenosis after left-sided failure.
Mitral stenosis
The most characteristic type of deformity causes mitral stenosis.
Mitral stenosis is the result of rheumatic endocarditis or bacterial endocarditis.
The gross appearance of the stenotic valve varies greatly according to the degree of
involvement.
Fibrous adhesion at the comissures may be slight or extensive.
The leaflets are fibrotic and thickened, especially toward the closing edges.
Contraction of scar tissue takes place, the valve leaflets become more rigid, and calcification
of the mitral cusps and ring frequently is present to a greater or lesser degree.
Ulceration of the thickest part of deformed valve is a common occurrence.
The orifice becomes considerably narrowed.
When the valves are less extensively involved and the bases of the leaflets are still somewhat
pliable, the narrowed opening is surrounded but puckered, thickened tissue, so-called
purse-string puckering. As the entire valve becomes more rigid it takes on appearance of a
fixed diaphragm with a narrow oval or curved opening, a “buttonhole” or “fish-mouth”
orifice.
The effects of mitral stenosis develop as a consequence of obstruction to the outflow of
blood from the left atrium and include the following:
a) Dilatation and hypertrophy of the left atrium, which occasionally appears as a huge
saclike structure (so-called giant left atrium).
b) Endocardial fibrous thickening of the left atrium.
c) Pronounced chronic passive congestion of the lungs; eventual pulmonary-arteriolar
thickening.
d) Hypertrophy and dilatation of the right ventricle as a result of pulmonary
hypertension
e) Dilatation of the right atrium as a right-sided heart failure develops.
96
f) A normal-sized left ventricle or, in prolonged mitral stenosis, atrophic left ventricle
caused by reduced inflow of blood, with possible hypertrophy of this ventricle if mitral
insufficiency or aortic stenosis is present.
One of the complications that may occur in mitral stenosis and the consequent atrial
dilatation is atrial fibrillation. Atrial fibrillation contributes to blood stasis and predisposes
to development of thrombosis, especially in the left atrial appendage; systemic embolism
may result.
Myocarditis
Rheumatic myocarditis is characterized by the presence of
1. Granulematous myocarditis. It is characterized by the presence of specific Aschoff bodies.
There is gradual subsidence of the inflammatory reaction, and the Aschoff bodies are
converted into small scars.
2. Nonspecific exudative interstitial myocarditis. It is characterized by diffuse or focal
lymphohistiocytic infiltration and vasculitis. In the later stages of the disease may diffuse
small-focal cardiosclerosis.
3. Parenchymal damage may lead to acute cardiac insufficiency and to death in early stages of
disease or to chronic ischemic heart disease.
Pericarditis
The tendency to affect serous membranes is one of the distinctive features of RF, and
fibrinous pericarditis is a prominent part of the picture of acute rheumatic heart disease.
The exudate varies from a thin film of fibrin to a shaggy coat with adhesions between the
layers of the pericardium, thus the designation of “shaggy” heart, or “cor villosum”.
Microscopically, fibrin is seen as a shaggy layer on the surface of the epicardium and an
infiltrate of lymphocytes, plasma cells, histiosytes, and occasionally neutrophils are present.
Subsequently, organization of the fibrin by vascularized connective tissue may be observed.
This may lead to fibrous thickening and adhesions of the visceral and parietal layers, to
partial or complete obliteration of the pericardial cavity, and to a “chronic adhesive
pericarditis”.
Although pericarditis may be the most prominent gross manifestation of the acute disease,
it is usually a little physiologic significance and does not usually affect the clinical course of
the patient.
Postmorten diagnosis of old rheumatic disease is based on the following marks:
1. Chronic adhesive pericarditis, especially circumscribed obliteration of the cardiac sac nears
the apex.
2. Fibrous thickening of the valve leaflets, especially at the line of closure.
3. Valvular deformities, especially aortic or mitral stenosis of insufficiency and, more
significantly, involvement of both the aortic and mitral valves.
4. Thickening, shortening, and adhesions of the chordal tendineae.
5. Microscopic changes, including foci of perivascular interstitial fibrosis and vascularization of
the valves.
6. The chief causes of death in RF patients are cardiac failure, infective endocarditis, and
embolism. Death may, however, be attributable to various conditions such as pneumonia.
RHEUMATOID ARTHRITIS
Rheumatoid arthritis (RA) is a chronic progressive inflammatory arthritis of unknown
origin involving multiple joints and characterized by disorganization of connective tissue of the
synovial membrane and articular cartilarge and development of their deformation. RA is likely an
autoimmune disease.
RA is basically a severe form of chronic synovitis that can lead to destruction and ankylosis
of affected joints. The small joints of hands and feet are usually the first and most common
to be involved, with lesions of the large joints appearing later in the course of the disease.
Although the skin, eyes, heart, lungs, spleen, lymph nodes, sceletal muscle, central and
peripheral nervous system, and other organs can be affected.
97
Females are affected three times more often than males and there is peak prevalence in the
third to fourth decades of life.
Pathogenesis of RA
Rheumatoid disease is often accompanied by characteristic immunoglobulin (often IgM),
called rheumatoid factosr (RF), in affected person serum. These factors are against the own
immunoglobulins (often IgG) and are of considerable complexity; they are capable of acting
as antiglobulins and of forming complexes with abnormal antigenic gammaglobulins in vivo
and in vitro.
RF forms locally in joint fluid; an immune complex binds complement and forms the intraarticular chemotactic factors C3a and C5a. The resultant accumulation of neutrophils
contributes to the pathogenesis of the joint disease.
Other autoantibodies are also found in RA, and in addition to circulating immune
complexes, cell-mediated immune systems also contribute to the pathogenesis of the
articular and extra-articular manifestations of RA.
The cells and mediators that likely play a role in the RA include neutrophils, synovial lining
cells, lymphocytes, and macrophages. The last-mentioned produce IL-1 and tumor necrosis
factor, cytokines known to stimulate release of collagenases and other lytic enzymes.
The trigger for these immunologic reactions remains unknown; some authors have
suggested Epstein-Barr virus (EBV) infection.
Morphology of RA
Main morphological appearance of RA is synovitis
RA generally first affects the small, proximal joints of the hands and feet, but then may involve,
usually symmetrically, the wrists, elbows, ankles, and knees.
Stages of synovitis:
1. First stage
Acute inflammatory reaction with development of edema, hyperemia, and infiltration by
small and large lymphocytes, plasma cells, plasmoblasts, mast cells, and macrophages,
indicating the presence of both humoral and cellular immune response arises.
There often are small areas of superficial necrosis of synovial lining cells with formation of
superficial erosions covered by fibrinoid deposits; these deposits are composed of fibrin and
small amounts of gamma globulin and complement components.
An exudate containing polymorphonuclear leukocytes, may with ingested immune
complexes, accumulates in joint cavity.
Not infrequently, 2 to 3 mm “rice” bodies, composed of fibrin, fibronectin, collagen and
immunoglobulin are present in joint cavities of seropositive patients.
2. Second stage
Hypertrophy of the synovium, synoviocytic hyperplasia, and an intense lymphoplasmacytic
and hystiocytic infiltrate take place.
Granulation tissue composed of synovial fibroblasts and capillaries causes grossly
recognizable villous thickening of the synovium, whose lining cells become hypertrophic
and hyperplastic.
In some of these lining cells as well as lymphocytes and plasma cells of the synovium and in
leukocytes of the synovial fluid occur.
This exuberant synovium is known as pannus, which eventually fills the joint space,
encroaching upon the articular surfaces.
Release of destructive enzymes (proteases and collagenases) and cytokines (particularly IL1) and pannus formation destroy cartilage, leading to changes very reminiscent of
degenerative joint disease.
3. Third stage
Fibrous and bony ankylosis can result.
As the pannus ages, vascularity decreases, the fibrosis and collagenization lead to shrinkage
of the capsule, progressive narrowing of the joint space, and displacement or increasing
approximation of the ends of the bones.
Closely opposing bones may become fused by bone bridges developing in the scar tissue, or
they may be telescoped into each other, with complete elimination of the joint.
Other features include rheumatoid nodules (or rheumatoid granuloma) in subcutaneous
tissues (areas of necrosis surrounded by palisade of fibroblasts and white cells at pressure
98
points such as elbows), acute vasculitis (in patients with high rheumatoid factors), and
nonspecific, fibrinous inflammatory lesions of lungs, pleura, pericardium, myocardium,
peripheral nerves, and eyes.
The most common extra-articular lesion
The most common extra-articular lesion is the subcutaneous nodule, a granuloma of a few
millimeters to several centimeters in size, developing usually in areas close to the joints and
subject to minor mechanical insults.
Vasculitis associated with deposition of immune complexes in vessel walls is seen especially
in patients with high serum titers of IgM-RF complex; occlusion of the vessel may result in
ischemia and microinfarcts. Occlusion of the large vessels can cause gangrene of the
terminal phalanges of fingers or toes.
Cardiac lesions may involve the pericardium, myocardium, and endocardium, with focal
accumulation of lymphocytes and plasma cells, vasculitis, granulomas, fibrosis, and
amyloidosis.
Pulmonary lesions may be focal and granulematous or diffuse, interstitial, or intraalveolar.
The result is focal fibrosis.
Lymph nodes show hyperplasia and, less commonly, granulomas. Several types of scleritis
and retinopathy have been described in about 1% of patients with rheumatoid disease.
Amyloidosis is a late complication of RA with data on the frequency varying from 25% to
60%.
Clinical features
Variable. Most patients experience a prodrome of malaise, fever, fatigue, and
musculoskeletal pain before joint involvement occurs.
The lucky patient experiences mild transient disease without sequelae, but most has
fluctuating disease with the greatest progression during the initial 4 to 5 years. In a
minority the onset in acute, with rapidly progressive development of joint deformities.
Characteristic deformities are radial deviation of the wrist with ulnar deviation of the
fingers.
Extra-articular manifestations (mentioned above), although infrequent, are rarely the
presenting features of the disease, and tend to develop in patients with high RF titers.
Some of the total morbidity of RA is caused by GI bleeding from long-term aspirin therapy,
infections from steroid use, or amyloidosis in long-term severe disease.
The death is caused by uremia.
SYSTEMIC LUPUS ERYTHEMATOSUS (SLE)
SLE (Libmnan-Sacks disease) is the classic prototype of the multisystem disease of
autoimmune origin, characterized by a bewildering array of autoantibodies, particularly antinuclear
antibodies. Acute or insidious in its onset, it is chronic, remitting and relasping, often febrile illness
characterized principally by injury to the skin, joints, kidney, and serosal membranes.
Likely most autoimmune diseases, SLE is predominantly a disease of women, with
frequency of 1 in 700 among women between ages of 20 and 64 and female to-male ratio of
9:1.
The cause of SLE remains unknown, but the existence of a seemingly limitless number of
antibodies in these patients against self-constituents indicates that the fundamental defect
in SLE is a failure of the regulatory mechanisms that sustain self-tolerance. Some authors
consider that RNA virus may cause it.
Antibodies have been identified against an array of nuclear and cytoplasmic components of
the cell that are either organ or species specific. Apart from their value in the diagnosis and
management of patients with SLE, these antibodies are of major pathogenetic significance,
as, for example, in the immune complex-mediated glomerulonephritis so typical of this
disease.
Antinuclear antibodies are directed against several nuclear antigens and can be grouped
into four categories: (1) antibodies to DNA, (2) antibodies to histones, (3) antibodies to
nonhistone proteins bound to RNA, and (4) antibodies to nuclear antigens.
SLE appears to be a complex disorder of multifactorial origin resulting from interactions
among genetic, hormonal, and environmental factors acting in concert to cause activation
of helper T cells and B cells that results in the secretion of several species of autoantibodies.
In this complex web, each factor may be necessary but not enough for clinical expression of
99
the disease; the relative importance of various factors may vary from individual to
individual.
Morphology
The morphologic changes in SLE are extremely variable, reflecting the variability of the clinical
manifestations and the course of the disease in individual patients. It can also be said that none of
these morphologic changes is pathognomic. The constellation of clinical, serologic, and morphologic
changes is essential for diagnosis.
SLE is characterized by different cellular and tissue changes which can be divided
into 5 groups:
1. Acute necrotic and degenerative changes of the connective tissue (all stages of
disorganization).
2. Subacute interstitial inflammation of all organs including nervous system with involvement of
microcirculation (capillaritis, arteriolitis, vasculitis).
3. Changes of sclerotic character caused by the above changes. This group is characterized by
onion-like sclerosis in the spleen.
4. Changes of the immune system. Focal accumulations of leukocytes with marked
plasmatization are present in the central and peripheral organs. Macrophagic activity is
increased.
5. Nuclear pathology in the cells of all organs and tissues, particularly in the lymph nodes. The
shape of the nuclei does not change but they gradually lose DNA and look pale after stain.
After the death of the cell, the nucleus disintegrates into granules, i.e. hematoxylin bodies.
This phenomen characterizes LSE. Neutrophils and macrophages phagocytize hematoxylin
bodies and form so-called “lupus cells”. Their presence in the blood is a significant sign of
SLE. Except for the blood, they can be found in the bone marrow, spleen, lymph nodes and the
vascular walls.
Visceral manifestations of LSE
The most characteristic lesions result from the deposition of immune complexes and are found
in the blood vessels, kidneys, connective tissue, and skin.
An acute necrotizing vasculitis involving small arteries and arterioles may be present in any
tissue although skin and muscles are most commonly affected. Fibrinoid deposits
characterize the vessel walls of arteries. In chronic stages, vessels undergo fibrous
thickening with narrowing lumen. In the spleen, these vascular lesions involve the central
arteries and are characterized by marked perivascular fibrosis, producing so-called
“onion-skin” lesions.
Kidney. On light microscopic examination, the kidney appears to be involved in 60 to 70%
of cases, but if immunofluorescence and electron microscopy are included in the
examination of biopsy material, almost all cases of SLE show some renal abnormality.
According to WHO morphologic classification of lupus nephritis, five patterns are
recognized:
1. Normal by light, electron, and immunofluorescent microscopy (class 1), which is quite
fare.
2. Mesangial lupus glomerulonephritis (class 2).
3. Focal proliferative glomerulonephritis (class 3).
4. Diffuse prolirefative glomerulonephritis (class 4).
5. Membranous glomerulonephritis (class 5).
It should be noted, however, that none of these patterns are specific for lupus.
Skin. The skin is involved in the majority of patients. Characteristic erythema in the bridge
of the nose and cheeks (facial “butterfly”) occurs, but a similar rash may also be seen on
the extremities and trunk. Urticaria, bullae, maculopapular lesions, and ulcerations also
occur. Exposure to sunlight incites or accentuates the erythema. Histologically, the involved
areas show liquefactive degeneration of the basal layer of the epidermis together with
edema at the dermal junction. In the dermis, there is variable edema and perivascular
mononuclear infiltrates. Vasculitis with fibrinoid necrosis of the vessels may be prominent.
Joints. Joint involvement is frequent, the typical lesion being a nonserosive synovitis with
little deformity. The latter fact distinguishes this arthritis from that seen in rheumatoid
100
disease. In the acute phases of arthritis in SLE, there is exudation of neutrophils and fibrin
into the synovium and a perivascular mononuclear cell infiltrate in the synovial tissue.
Serosal cavities. Inflammation of the serosal lining membranes may be acute, subacute,
or chronic. During the acute phase, the mesothelial surfaces are sometimes covered with
fibrinous exudate. Later they become thickened, opaque, and coated with a shaggy fibrous
tissue that may lead to partial or total obliteration of the serosal cavity.
Cardiovascular system. Involvement is manifested primarily in the form of pericarditis.
Valvular endocarditis may occur, but it is clinically insignificant. In the era before the
widespread use of steroids, so-called Libman-Sacks endocarditis was more common.
The nonbacterial verrucous endocarditis takes the form of single or multiple irregular 1-to3mm warty deposits on any valve in the heart, distinctively on either surface of the leaflets.
Myocarditis, manifested as nonspecific mononuclear cell infiltration, may also be present
but is less common.
Spleen. The spleen may be moderately enlarged.
Lungs. In lungs the pneumanitis, fibrosing alveolitis and diffuse interstitial fibrosis are
found out.
The most common causes of death are renal failure (uremia) and intercurrent infections,
followed by diffuse central nervous system disease. Patients treated with steroids and
immunosuppressive drugs incur the usual risks associated with such therapy.
BECHTEREW’S DISEASE
Bechterew's disease is a chronic disease involving joints and ligaments of the spine
causing its immobility. Involvement of peripheral joints and inner organs is possible.
Etiology: infectious-allergic factor, spine injury and hereditary factors. The disease mainly
occurs in men with antigen histocompatibility HLA-B27.
Morphology. Destructive inflammatory changes in the tissues of small joints of the spinal
column resembling those in rheumatoid arthritis with destruction of articular cartilages,
growth of stroma in the cavity of the joint and its-bony metaplasia with development of
bone ankylosis and limitation of their mobility. The same process with bone formation
develops in intervertebral disks, which results in complete immobility of the spinal column.
Visceral changes: chronic inflammation and sclerosis of aorta, heart, lungs; amyloidosis in
kidneys.
SYSTEMIC SCLERODERMA
Systemic scleroderma is chronic disease with skin involvement and visceral
manifestations.
Etiology. Viruses, genetic factors causing disturbances in collagen synthesis cannot be
excluded. Abnormal collagen disintegrates quickly and is followed by sclerosis.
Morphology. All stages of connective tissue disorganization against the background of
slight cellular reaction are noted in the skin and internal organs. The condition results in
sclerosis and hyalinosis. The skin becomes dense; its mobility is poor. Cortical necrosis may
develop when the vessels of the kidneys are involved. It manifests by acute renal failure,
termed “true sclerodennic kidney”. Development of large-focus cardiosclerosis, fibrosis of
the lungs and subpleural cavities (basal pneumosclcrosis) are possible.
The complications and the causes of death depend on the visceral lesions (kidneys, heart,
lungs).
DERMATOMYOSITIS
Dermatomyositis is a chronic rheumatic disease involving striated and in rare cases
smooth muscles and skin. If the skin is not damaged, the disease is called polymyositis. It
may occur at any age, mainly in women.
Morphology. Striated muscles, the muscles of the pharynx, larynx, and diaphragm, ocular
muscles are involved. Degeneration, calcinosis, necrosis, edema, cellular reactions are
observed. Degenerative, inflammatory and sclerotic changes are observed in the heart,
lungs, and alimentary tract. Hyperplasia against the background of plasmatization is
observed in the immune organs.
Clinico-morpholbgical forms:
1) Primary (idiopathic). Primary form in children is caused by genetic factor.
2) Secondary (tumor). Secondary form is frequently observed in cancer of ovaries,
stomach, lungs, and breast.
101
Each form may be acute, subacute, constantly relapsing and chronic.
DISEASES OF RESPIRATORY SYSTEM
ACUTE BACTERIAL INFECTIONS OF THE LUNGS
Occur when normal lung or systemic protective mechanisms are impaired. Pulmonary
protective mechanisms include nasal, tracheobronchial, and alveolar mechanisms to filter,
neutralize, and clear inhaled organisms and particles.
Important factors interfering with normal lung defenses are
1. Decreased cough reflex leading to aspiration (seen in coma, anesthesia, drug effects).
2. Injury to mucociliary apparatus (as with cigarette or other smoke / gaseous
inhalations).
3. Decreased phagocytic /bactericidal function of the alveolar macrophage (as a result
of alcohol, tobacco, oxygen toxicity).
4. Edema/congestion (CIHD).
5. Accumulation of secretions.
There are a lot of diseases, of pulmonary system as well as the etiologic factors, which cause
these diseases. Acute and chronic bronchitis, pneumonia, destructive processes (abscess
and gangrene), bronchial asthma, chronic non-specific pulmonary diseases and cancer of
lungs are the most common.
Pathogenic organisms gain access to the lung through the airways, through the
bloodstream, by traumatic implantation, or by direct spread across the diaphragm from the
subphrenic source, probably through the lymphatics. The most common route is the
airways.
Pneumonias
Pneumonia is acute inflammation of the respiratory tract with deposition of intraalveolar
exudates.
Etiologic classification of pneumonia:
1. Bacterial pneumonia.
2. Viral and mycoplasmal pneumonia.
3. Other types of pneumonias:
a) Pneumocystis carini pneumonia.
b) Legionella pneumonia.
c) Aspiration pneumonia.
d) Hypostatic pneumonia.
e) Lipid pneumonia.
Clinical-morphological classification:
1. Lobar pneumonia.
2. Bronchopneumonia (lobular pneumonia).
3. Interstitial pneumonia.
Lobar pneumonia
Synonyms: crupous, lobular, fibrinous, pleurapneumonia.
Croupous pneumonia is infectious-allergic infection and involves a lobe of lung.
Most lobar pneumonias are caused by pneumococci and Klebsiella pneumonia which enter
the lungs via the airways.
The pneumococcus continues to be responsible for 30% to 80% or more of communityacquired pneumonias.
Groups at particular risk include the very young and very old, alcoholics, diabetics,
spleenectomized subjects, and patients with multiple myeloma or circle cell disease.
Hypersensivity of immediate type plays an important role in pathogenesis.
Pleural involvement occurs commonly in lobar pneumonia Pneumococcal pneumonia
typically presents the picture of lobar pneumonia. One or occasionally several lobes of the
lung are involved. Fibrinous exudates in alveoli are presence.
Traditionally the progress of the disease is divided into four stages:
1. Congestion and Edema predominates in the first 24 hours. The initial response to the
organism is edema, which spreads throughout the lobe through pores of Kohn and
102
2.
3.
4.
1.
2.
3.
4.
5.
bronchioles. At this stage an involved lobe appears distended, moist, and deep red of
purple. The pleura are shiny, and fluid exudes from the cut surface.
Red hepatization (2 days) describes lung tissue with confluent acute exudate containing
neutrophils and red cells, giving a red, firm. Lobe is liver-like.
Gray hepatization (4-6 days) follows, as the red cells disintegrate and the remaining
fibrinous-suppurative exudates persist, giving a gray-brown gross appearance.
Resolution (9-11 days) is the favorable final stage in which consolidated exudates
undergoes enzymatic and cellular degradation and clearance. Normal structure is
restored.
Complications:
Carnification is organization of fibrinoid exudate.
Abscess formation. Lung abscess results from the breakdown of alveolar walls.
Empyema (spread of infection to pleural cavity).
Gangrene.
Bacteremic spread leads to purulent meningitis, bacterial endocarditis, arthritis,
pericarditis and other organs.
Causes of death are acute cardiac-respiratory insufficiency and purulent complications.
Bronchopneumonia (focal pneumonia)
Bronchopneumonia is marked by patchy exudative consolidation of lung parenchyma
Polyetiologic. The most often agents are bacterias: pneumococci, staphylococci,
streptococci, hemophylus influenzae, pseudomonas aeruginosa, and coliform bacteria.
Bronchopneumonia often arises due to autoinfection. Depending pathogenesis
autoinfectional bronchopneumonia may be aspirationous, hypostatic, postoperative,
immunodificiency.
Bronchopneumonia often is a complication of others disease.
According to extent may be acynous, lobular, segmental, and miliary.
Morphology
Initially bronchi are affected. Then, inflammation spreads to parenchyma of lungs with
accumulation of exudates in the alveoli.
Grossly, the lungs show dispersed, elevated, focal areas of palpable consolidation and
suppuration.
Histological features consist of acute (neutrophilic) suppurative, serous, hemorrhagic or
mixed exudates filling airspaces and airways, usually about bronchi and bronchioles.
Outcomes and complications: resolution of the exudates usually restores normal lung
structure, but organization may occur and result in fibrous scarring in some cases.
Aggressive disease may produce abscess, pleurisy, and empyema.
Streptococcal pneumonia
Beta-hemolytic streptococci are an uncommon cause of pneumonia at the present time. In
adults streptococcal pneumonia like other pneumonias usually occurs in elderly, severely
debilitated patients. Diabetes is also a risk factor. Infections caused by this microbe in the
newborn are discussed elsewhere.
The lower lobe is usually the site of major involvement.
The airways appear thickened and are filled with a hemorrhagic or purulent exudate.
The pneumonia is lobular with consolidated patches clearly centered on terminal
bronchioles. The distinctive microscopic feature of streptococcal pneumonia is greater
interstitial involvement than in other bacterial pneumonias. There is necrosis of the
epithelium of distal airways with infiltration of the bronchial walls by neutrophils and
mononuclear cells. The interstitial infiltrate also extends into the adjacent alveolar walls.
Staphylococcal pneumonia
Staphylococcal pneumonia usually occurs either in the presence of a source of bacteremia
or after viral infection.
Hematogenous pneumonia is seen in those with soft-tissue infections, in patients
undergoing long-term dialysis. The lesions may appear as septic infarcts that are yellow and
103
purulent but preserve to some degree the wedge-shaped configuration of infarcts and are
associated with thrombosed vessels, or they may be rounded patches of necrotizing
pneumonia that break down, giving rise to abscesses.
Staphylococcal pneumonia also results from spread of organisms from the colonized
nasopharynx. The lesions are those of bronchopneumonia accompanied by a hemorrhagic
and necrotizing bronchitis. Purulent exudate fills the bronchioles and spreads into the
adjacent acini.
Staphylococcal bronchopneumonia is not rare in children less than 6 months of age. A
notable feature of staphylococcal pneumonia in small children is development of abscesss.
Local complications of staphylococcal pneumonia include empyema and bronchopleural
fistula.
Aspiration pneumonia
Aspiration pneumonia results from inhaling different agents into the lungs. These substances
include food, gastric contents, infected material from oral cavity, amniotic fluid or meconium in
infants, etc.
Hypostatic pneumonia
Hypostatic pneumonia is the term used for the collection of edema fluid and secretions in the
dependent parts of the lungs in bed-patients.
Interstitial pneumonia
Infections by viruses, mycoplasma pneumonia, pneumocystis carinii, etc. result in varied
clinical and pathologic patterns, ranging from relatively mild upper respiratory tract involvements to
severe lower respiratory tract disease.
Morphology
Patchy or lobar areas of congestion without the consolidation of bacterial pneumonias.
A predominance of interstitial pneumonitis with widened, edematous alveolar walls
containing a mononuclear inflammatory cell infiltrates.
The formation of hyaline membranes, reflecting diffuse alveolar damage.
Pneumocystic pneumonia is characterized by desquamation of alveolar epithelium. Alveoli
filled by foamy fluid and pneumocysts, and also hyperemia and inflammatory infiltration of
the alveolar septs. It may pattern in AIDS.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
The term Chronic Obstructive Pulmonary disease (COPD) refers to a group of conditions that
share a major symptom – dyspnea - and are accompanied by chronic or recurrent obstruction to air
flow within the lung.
Obstructive diseases are characterized by increased resistance to airflow because of chronic
or recurring expiratory obstruction.
In their prototypical forms, these individual disorders – chronic bronchitis, bronchiectasis,
asthma, emphysema – have distinct anatomic and clinical characteristic.
Hypertension of pulmonary circulation and “cor pulmonale” develops in all Chronic
Obstructive Pulmonary diseases.
Amyloidosis of kidneys and chronic renal insufficiency may develop often.
Death of the most patients with COPD is due to
1) Respiratory acidosis and coma,
2) Right-sided failure,
3) Massive collapse of the lung secondary to pneumothorax.
Chronic Bronchitis
The widely accepted definition of chronic bronchitis is a clinical one – chronic bronchitis
(CB) is present in any patient who has persistent cough with sputum production for at least 3 months
in at least 2 consecutive years.
This disorder, so common among habitual smokers and inhabitants of smog-laden cities, is
not nearly so trivial as was once thought.
104
The role of infection appears to be secondary. It is not responsible for the initiation of CB
but is probably significant in maintaining it and may be criterial in producing acute
exacerbations.
Pathogenesis. Two sets of factors are important in the genesis of chronic bronchitis:
1. Chronic irritation by inhaled substances.
2. Microbiologic infections.
Morphology
The hallmark and earliest failure of CB is hypersecretion of mucus in the large airways, and
is associated with hypertrophy of the submucosal glands in the trachea and bronchi.
As CB persists, there is also a marked increase in goblet cells of small airways – small
bronchi and bronchioles – leading to excessive mucus production that contributes to airway
obstruction.
Although mucus hypersecretion in large airways is the cause of sputum overproduction, it is
now thought that accompanying alterations in the small airways of the lung can result in
physiologically important and early manifestations of the chronic airway obstruction.
Histological features of the small airways:
1. Goblet cell metaplasia with mucus plugging of the lumen.
2. Clustering of pigmented alveolar macrophages.
3. Inflammatory infiltration.
4. Fibrosis of bronchiolar wall.
Outcomes and complications
Lead to “cor pulmonale” and heart failure.
Cause atypical metaplasia and dysplasia of the respiratory epithelium, providing a possible
soil for cancerous transformation.
Amyloidosis of kidneys.
Lead to bronchiectasis.
Bronchiectasis (BE)
BE is chronic necrotizing infection of the bronchi and bronchioles leading to or associated with
abnormal dilation of these airways.
BE has many origins and usually develops in association with following conditions:
1. Bronchial obstruction, due to tumor, foreign bodies, and occasionally mucous impaction, in
which the BE are localized to the obstructed lung segment; or due to diffuse obstructive
airway disease, most commonly atopic asthma and chronic bronchitis, measles.
2. Congenital or hereditary conditions, including congenital BE, cystic fibrosis, intralobar
sequestration of the lung states, and immune cilia and Kartagener’s syndromes.
3. Necrotizing pneumonia, most often caused by tubercle bacillus or staphylococci or mixed
infections.
Morphology
BE usually affects the lower lobes bilaterally, particularly those air passages that is most
vertical, and is most severe in the more distal bronchi and bronchioles.
When tumors or aspiration of foreign bodies leads to BE, the involvement may be sharply
localized to a single segment of the lungs.
The pleura is usually fibrotic and thickened with adhesions to the chest wall. Cut surface
has honey-combed appearance. The walls of bronchi are thickened and the lumen arc filled
with mucus.
The airways are dilated; sometime up to four times normal size. These dilations may
produce:
1. Long, tube-like enlargements (cylindroid BE) in 1 to 4 type of bronchus.
2. May cause fusiform or even sharply saccular distention (saccular BE) in 6-10 types of
bronchus.
The histologic findings vary with the activity and chronicity of the disease:
1. In the full-down active case, there in an intense acute and chronic inflammatory
exudation within the walls of bronchi and bronchioles, associated with desquamation
of the lining epithelium and extensive areas of necrotizing ulceration. There may be
squamous metaplasia of the remaining epithelium.
105
2. In some instances, the necrosis completely destroys the bronchial or bronchiolar
walls and forms a lung abscess.
3. Fibrosis of the bronchial and bronchiolar walls and peribronchial fibrosis develop in
the more chronic cases.
Outcomes and complications
1. Obstructive ventilatory insufficiency can lead to marked dyspnea and cyanosis.
2. Pulmonary hemorrhage.
3. Pulmonary abscess.
4. Empyema of the pleura.
5. Metastatic brain abscess.
6. “Cor pulmonale” and chronic cardiac-pulmonary insufficiency.
7. Amyloidosis are less frequent complications of BE.
Emphysema
Emphysema is a condition of the lung characterized by abnormal permanent enlargement of
the airspace distal to the terminal bronchiole, accompanied by destruction of their walls, and without
obvious fibrosis. In contrast, the enlargement of airspaces unaccompanied by destruction is termed
overinflation, for example, the distention of airspaces in the opposite lung following unilateral
pneumonectomy.
Pathogenesis
While details of the genesis of the two common forms of emphysema – centiacinar and
panacinar – remain unsettled, the most plausible hypothesis to account for the destruction of alveolar
walls is the protease-antiprotease mechanism. Thus, emphysema is seen to result from the destructive
effect of the high protease activity in subjects with low antiprotease activity.
The protease-antiprotease hypothesis also explains the deleterious effect of cigarette smoking.
1. Smokers have greater numbers of neutrophils and macrophages in their alveoli. The increased
recruitment of neutrophils into the lung is likely to result, in part, from the release by
activated alveolar macrophages of neutrophil chemotactic factors, this release being
stimulated by smoking. In addition, nicotine is chemotactic for neutrophils, and cigarette
smoke activates the alternative compliment pathway.
2. Smoking stimulates release of elastase from neutrophils.
3. Smoking enhances elastolytic proteases activity in macrophages; macrophage elastase is not
inhibited by alpha-1 –AT and, indeed, can proteolytically digest this enzyme.
4. Oxidants in cigarette smoke and oxygen free radicals secreted by neutrophils inhibit alpha-1AT and thus decrease net antielastase activity in smokers.
It is thus postulated that impaction of smoke particles in the small bronchi and bronchioles,
with the resultant influx of neutrophils and macrophages, and increased elastase and decreased
alpha-1-AT activity causes to the centriacinar emphysema seen in smokers.
Classification
Although the term “emphysema” is sometimes loosely applied to diverse conditions, there are
four types:
1. Centriacinar (cenrolobular) emphysema. The distinctive feature of this type is the pattern of
involvement of the lobules; the central or proximal parts of the acini, formed by respiratory
bronchioles, are affected, whereas distal alveoli are spared. The walls of the emphysematous spaces
often contain large amount of black pigment. Inflammation around bronchi and bronchioles and in
the septa is common. Moderate-to-severe degrees of emphysema occur predominantly in heavy
smokers, often in association with chronic bronchitis. In addition, some lesions of so-called coal
workers’ pneumoconiosis bear a striking resemblance to centriacinar emphysema.
2. Panacinar (panlobular) emphysema. In this type the acini are uniformly enlarged from the level at
the respiratory bronchiole to the terminal blind alveoli. This type of emphysema is associated with
alpha-1-antitrypsin deficiency.
3. Paraseptal (distal acinar) emphysema. In this type the proximal portion of the acinus is normal,
but the distal part is dominantly involved. The emphysema is more striking adjacent to the pleura,
along the lobular connective tissue septa, and at the margins of the lobules. This type of emphysema
probably underlies many of the cases of spontaneous pneumothorax in young adults.
106
4. Irregular emphysema, so named because the acinus is the irregularly involved, is almost invariably
associated with scarring. Thus, it may be the most common form of emphysema, as careful search of
most lungs at autopsy shows one or more scars from a healed inflammatory process. In most
instances, these foci of irregular emphysema are asymptomatic.
Types of emphysema according to cause
1. Compensatory E. This term is sometimes used to designate dilation of alveoli but not destruction of
septal walls in response to loss of lung substance elsewhere. It is best exemplified by the
hyperexpansion of the residual lung parenchyma that follows surgical removal of a diseased lung or
lobe.
2. Obstructive overinflation refers to the condition in which the lung expands because air is trapped
within it.
3. Senile E. refers to the overdistended, sometimes voluminous lungs found in the aged.
Bullous E. refers merely to at any form of E. that produces large subpleural blebs or bullae (spaces
more than 1 cm in diameter in the distended state).
4. Interstitial E. The entrance of air into the connective tissue stroma of the lung, mediastinum, or
subcutaneous tissue is designated interstitial emphysema.
Morphology
The diagnosis and classification of the emphysemas are based on naked eye (or hand lens)
examination of lungs fixed in a state of inflation.
Panacinar emphysema, when well developed, produces voluminous lungs, often
overlapping the heart and hiding it when the anterior chest wall is removed.
The macroscopic signs of centriacinar emphysema are less impressive. The lungs may not
appear particularly pale or voluminous unless the disease is well advanced.
Generally, the upper two-thirds of the lungs are more severely affected.
Large apical blebs or bulla are more characteristic of irregular emphysema secondary to
scarring.
Microscopical examination is accessory to visualize the abnormal fenestrations in the walls
of the alveoli, the complete destruction of septal walls, and the distribution of damage
within the pulmonary lobule. With advance of the disease, adjacent alveoli fuse, producing
even larger abnormal airspaces and possibly blebs or bulla. Often the respiratory
bronchioles and vasculature of the lung are deformed and compressed by the
emphysematous distortion of the airspaces, and, as mentioned, there may or may not be
evidence of bronchitis or bronchiolitis.
Bronchial Asthma (BA)
Bronchial asthma is a disease characterized by hyper-reactive airways, leading to episodic,
reversible bronchoconstriction, owing to increased responsiveness of the tracheobronchial tree to
various stimuli. A severe and unremitting type of the disease termed status asthmaticus may prove
fatal.
Pathogenesis
Chronic airway inflammation involving many cell types and inflammatory mediators
accompanies the bronchial hyper-responsiveness of asthma.
Nevertheless, the relationship of the inflammatory cells and their mediators to airway
hyper-reactivity is not fully understood.
Classification
1. Extrinsic BA is initiated by a type 1 hypersensitivity reaction induced by exposure to an extrinsic
antigen. Subtypes include atopic (allergic), BA, occupational BA (many forms), and allergic
bronchopulmonary aspergillosis (bronchial colonization with aspergillus organisms followed by
development of IgE antibodies).
2. In contrast, intrinsic BA is initiated by diverse, nonimmune mechanisms, including aspirin,
pulmonary infections; especially those caused by viruses, cold, inhaled irrigants (pollutants such as
sulfur dioxide), stress, and exercise.
Morphology
107
The morphologic changes in asthma have been described principally in patients dying of status
asthmaticus, but it appears that the pathology in nonfatal cases is similar.
Grossly, the lungs are overdistended because of overinflation, and there may be small areas
of atelectasis.
The most striking macroscopic finding is occlusion of bronchi and bronchioles by thick,
tenacious mucous plaques.
Histologically, the mucous plaques whorls of shed epithelium, which give, rise to the wellknown Curschmann’s spirals.
Numerous eosinophils and Charcot-Leyden crystals are present.
The other characteristic histologic findings of BA include:
- Thickening of the basement membrane of the bronchial epithelium.
- Edema and inflammatory infiltrate in the bronchial walls, with a prominence of
eosinophils, which form 5 to50% of the cellular infiltrate.
- An increase in size of the submucosal glands.
- Hypertrophy of the bronchial wall muscle, a reflection of prolonged
bronchoconstriction.
- Emphysematous changes sometimes occur, and if chronic bacterial infection has
supervened, bronchitis may occur.
The classic asthmatic attack lasts up to several hours and is followed by prolonged
coughing; the raising of copious mucous secretions provides considerable relief of the respiratory
difficulty. In some patients, these symptoms persist at a low level all the time. In its most severe form,
status asthmaticus, the severe acute paroxysm persists for days and even weeks, and, under these
circumstances, ventilatory function may be so impaired as to cause severe cyanosis and even death.
Chronic Lung Abscess (LA)
The term “LA” describes a local suppurative process within the lung characterized by necrosis
of lung tissue.
Oropharyngeal surgical procedures, bronchial infections, dental sepsis, and bronchiectases
play important roles in their development.
The causative organisms are introduced by the following mechanisms:
- Aspiration of infective material.
- Antecedent primary bacterial infection.
- Septic embolism.
- Obstructive tumors.
- Direct traumatic punctures.
- Miscellaneous.
When all these causes are excluded, there are still cases in which no reasonable basis for the
LA formation can be identified. These are referred to as “primary cryptogenic” LA.
Morphology
Abscesses vary in diameter from lesions of a few millimeters to large cavities of 5 to 6 cm.
They may affect any part of the lung and may be single or multiple.
The cavity may or may not be filled with suppurative debris, depending on the presence or
absence of a communication with one of the air passages.
When such communications exist, the contained exudate may be partially drained to create
an air-containing cavity.
Superimposed saprophytic infections are prone to flourishing within the already necrotic
debris of the abscess cavity.
Continued infection leads to large, fetid, green-black, multilocular cavities with poor
demarcation of their margins, designated gangrene of the lung.
The cardinal histologic change in all abscesses is suppurative destruction of the lung
parenchyma within the central area of cavitation.
A reactive fibrous wall often surrounds chronic abscesses.
Complications include extension of the infection into the pleural cavity, hemorrhage, the
development of brain abscesses or meningitis from septic emboli, and rarely reactive secondary
amyloidosis.
Idiopathic Pulmonary Fibrosis
108
Diffuse interstitial fibrosis occurs as a result of different pulmonary diseases. It is so called
“idiopathic pulmonary fibrosis” or “cryptogenic fibrosing alveolitis” or “chronic interstitial
pneumonitis”
Morphology
Pathological changes are bilateral and widespread.
Macroscopically the lungs are dense, reduced volume.
Honey-combing (i.e. enlarged, thick-walled air spaces) develops in parts of lung.
Microscopically, changes vary according to the stage of the disease with formation of
hyaline membranes.
There is edema and cellular infiltrate in the alveolar septa in early stage.
There is organization of the alveolar exudate and replacement fibrosis in the alveoli and in
the interstitial septal wall with variable amount of inflammation in advanced stage.
DISEASES OF ALIMENTARY SYSTEMS
Tonsillitis
Tonsillitis is infectious disease and is characterized by inflammatory changes in the crypts
of the adenoids and the tonsils on the anterior wall of the pharynx.
Tonsillitis can be acute and chronic.
Etiophathogenesis
Infectious agents are staphylococcus, streptococcus, adenovirus and bacterium’s
assotiations.
Transephithelial, hematogenic pathways are responsible for the transmission. Autoinfection
is most often cause of tonsillitis against a background the cooling and trauma
Acute edema and erythema, and sometimes purulent exudates and abscesses, may develop
in the crypts of the adenoids and the tonsils on the anterior wall of the pharynx.
Lymphoid tissue is distributed on the posterior pharyngeal wall and under the tongue, but
not as masses of nodes with crypts such as composes the palatine adenoids and facial
tonsils.
Tonsils and adenoids are usually unapparent in early infancy, but gradually undergo
hypertrophy and hyperplasia to rich their relatively greatest mass between 2 and 5 years of
age.
Their location is such that they are exposed to inspired air and food and whatever antigens
may be carried in either one. These tissues are part of the bursal system of immunity and
consist mostly of B cells.
Classification
Acute tonsillitis:
Cattharal tonsillitis is characterized by hyperemia and serous or mucous leucocytic
infiltration.
Fibrinous tonsillitis the deposition of whitish-yellowish fibrinous films (in diphtheria)
occurs.
Purulent tonsillitis is characterized by phlegmonous inflammation or the formation of
abscesses. Tonsils are enlarged due to edema and leucocytic infiltration.
Follicular tonsillitis is characterized by hyperplasia of tonsils. Leucocytic infiltration and
necrosis of follicles take place. Tonsils are enlarged and hyperemic.
Cryptous tonsillitis is characterized by the deposition of the serous, mucous or purulent
exudates, which are located in crypts.
Necrotic tonsillitis the ulceration occurs and can be in leukemia and scarlet fever.
Gangrenous tonsillitis the ulceration and hemorrhages occurs also.
Chronic tonsillitis
Chronic tonsillitis is characterized by the persistence of infection or due to relapse of acute
tonsillitis.
Hyperplasia and sclerosis of lymphoid tissue, sclerosis of tonsil’s capsule, increasing of
crypts, ulceration of the epithelium are morphological features of chronic tonsillitis.
109
Chronic infection can present as anorexia, failure to gain weight, low-grade fever, or
recurrent sore throats with high fever. Hypertrophy can be considerable and lead to mouth
breathing, or even upper airway obstruction, retention, sleep apnea, and, rarely, cor
pulmonale.
Persistent anterior and posterior cervical adenopathy in the absence of generalized
lymphadenopathy is evidence of chronic or recurrent infection. Inspection of the tonsils is
of limited help during acute episode.
Complication of tonsillitis
Extension of tonsillar infection can take place in the surrounding tissues and is called
peritonsillar abscess or quinsy. The retropharyngeal nodes drain both the adenoids and the
nasopharynx and can become chronically infected. This is known as retropharyngeal
abscess.
These complications of tonsillitis are usually caused by B-hemolytic streptococci, which are
almost sensitive to penicillin. Consequently, the widespread use of antibiotics to treat
streptococcal pharingitis has been associated with less suppuration in the peritonsillar of
retropharyngeal spaces.
Peritonsillar cellulitis and abscess are characterized by an extremely sore throat and often
high fever. If the condition is untreated, it may lead to significant swelling and even
occlusion of the oral pharynx.
Retropharyngeal abscess is virtually limited to infants in the first 2 years of life. The
characteristic findings and fever, hyperextension of the neck, dysphagia, and noisy
respirations. There is prominence of the infected pharyngeal wall but swelling is almost
always unilateral.
The importance of this disease is that it is commonly a precursor of rheumatic fever or one
form of glomerulonephritis.
Gastritis
Gastritis is an inflammation of gastric mucosa and can be acute and chronic.
Acute gastritis
Acute inflammation develops due to injury of the mucosa by the alimentary, drugs, toxic and
bacterial agents.
Morphologic classification of acute gastritis:
1.
2.
3.
4.
5.
6.
Catarrhal gastritis.
Fibrinous gastritis.
Phlegmonous gastritis.
Necrotic (or Corrosive).
Hemorrhagic gastritis.
Pseudomembranous.
Chronic gastritis
Chronic inflammatory changes in the mucosa of the stomach, with various degrees of loss of
the specialized glandular tissue, are extremely common, although often clinically silent.
Collectively constitute a morphologic continuum of increasingly intense inflammation of
mucosa accompanied by progressively more marked atrophy of the mucosa glands.
Glandular atrophy is often accompanied by metaplasia, dysplasia and atypia of the surface
epithelium.
Our understanding of the etiology and mechanism of gastritis and gastroduodenal
ulceration has been radically altered by the discovery of specific infective agent Helicobacter
pylori.
The Sidney System is a new classification based on this recent new knowledge. It
incorporates two separate divisions: histological and endoscopic.
Clasification
The histological classification incorporates three main positions:
1. Etiology.
110
2. Topography (i.e.-site affected: antrum, body or both).
3. Morphology (including information about activity, intestinal metaplasia - graded as
mild, moderate or severe).
The three main types of chronic gastritis are examples of this classification according to
topography use:
I. Autoimmune associated chronic pangaslritis with severe atrophy (Type A fundal gastritis)
Associated with circulating antibodies to parietal cells and intrinsic factor and complete loss
of parietal cells.
Loss of parietal cells leads to hypo- or achlorhydria, hypergastrinemia, inadequate synthesis
of intrinsic factor and vitamin B12 absorption.
Overt pernicious anemia develops in 10%.
Associated with Hashimoto’s thyroiditis and Addison’s disease, hence the term
autoimmune gastritis.
Intestinal metaplasia and dysplasia may occur and possibly resulting in gastric carcinoma.
II. Helicobacter pylori associated chronic gastritis of the antrum with moderate activity (Type B
gastritis)
The most common form of gastritis in all age groups.
Background factors are environmental such as intoxication, abnormal dietary, and alcohol.
Associated with gastric atrophy, intestinal metaplasia, gastric polyps, and gastric cancer.
Initially superficial, gradually becomes deeper to affect the entire mucosa with glandular
atrophy, leading to “chronic atrophic gastritis”.
Colonization of mucous layer and surface of mucosal cells with curved organisms, with little
to no tissue invasion, confined to areas of gastric mucosa.
Small foci of neutrophils, some passing to surface or into superficial crypt lumen occur,
superimposed on a variable background of chronic gastritis (active chronic gastritis with
abundant neutrophils).
III. Reflux-gastritis (formerly known as Type C gastritis)
Associated with reflux of duodenal contents in stomach.
May occur after gastric surgery, or with weakened pyloric sphincter tone.
Localization is antrum.
Achlorhydria and hypergastrinemia is absent.
According to topography
1. Antral gastritis.
2. Fundal gastritis.
3. Pangastritis.
According to morphology
Chronic superficial gastritis (early stage): lymphocytes and plasma cells in the upper third
of the lamina propria, some mucosal flattening.
Chronic atrophic gastritis (later stage): flattening of rugal folds, mucosa thinned and
flattened, chronic inflammation of full thickness of the mucosa, loss of glands, metaplasia of
mucosa to the intestinal type.
Peptic Ulcer Disease
Ulcerative disease is chronic disease with development chronic recurrent peptic ulcer.
Background factors:
Age. Often diagnosed in middle-aged to elder adults, but may appear in young adult life.
Common in industrialized nations.
Sex. Mail-female ratio 3:1.
Familial tendency and genetic factors for duodenal ulcer.
Environmental and geographical factors.
Dietary habits.
The ingestion of drugs (especially aspirin, corticosteroids).
Stresses may be important.
Cigarette smoking and alcohol.
Pathogenesis
Hypersecretion of gastric juice and emotional factors have been considered to be important in
the pathogenesis of peptic ulcers. The gastroduodenal mucous membrane is protected against
digestion of normal gastric secretions not only by its mucus coating but also by dilution and
111
neutralization with swallowed food, saliva, and regurgitated duodenal fluids. This is considered to be
the result of vagal stimulation and can be abolished by section of the vagus nerve. The spiral
bacterium Helicobacter (campylobacter pylori) has been frequently isolated from patients with
gastritis or peptic ulcer disease, but its pathogenic role remains to be determined. Prostaglandin
deficiency has also been implicated as a possible cause of peptic ulcer disease.
For duodenal ulcers, the most important cause is excess exposure to acid and pepsin. Major
influences for duodenal ulcers:
Hypertone of vagus with increasing of acid-peptic factors.
Abnormally rapid gastric emptying, exposing the duodenum to a greater acid load.
Increasing of the level of ACTG and glucocorticoids.
Duodenal ulcer has been associated with tension, stress, and anxiety but this is by no means
always the case and there is no agreement on the importance of stress in its pathogenesis.
For gastric ulcers, breakdown in mucosal defenses appears to be most important. Major
influences for gastric ulcers:
Suppression of hypothalamic and hypophysic functions.
Hypotone of vagus and decreasing of gastric secretion.
May involve decreased pyloric sphincter tone, and reflux of bile acids.
Weakening of protective factors of gastric mucosa.
Exogenous agents that damage the mucosa are more likely to cause gastric ulcers than
duodenal ulcers (alcohol, drugs, chemical substance).
Possible defect in gastric mucus with the presence of Helicobacter.
Morphogenesis and morphology
I. Erosion is superficial necrosis of mucosal epithelial elements.
These are tiny ulcers, a few millimeters in diameter, which are formed by the digestion of
the mucosal membrane overlying small hemorrhage.
They are usually multiple and affect all parts of the stomach.
They occur mostly on the apex of mucosal folds and involve only the mucosa.
Note that the changes are superficial so that restoration to normal can very quickly occur.
II. Acute ulcer
Loss of tissue penetrating into the submucosa.
Location: single or multiple lesions throughout the stomach and duodenum.
Circular and small, less than 1 cm in diameter.
Inflammatory reaction absent initially, develops secondarily.
Massive hemorrhage may be fatal.
Perforation can lead to peritonitis.
This type of ulcer usually heals without a visible scar.
III. Chronic peptic ulcer
The term “chronic” is applied when the pathological changes have penetrated and destroyed
the muscle coat; they are also, of course, of much longer duration than acute ulcers.
Gastric ulcers are located along the distal lesser curvature, usually within about 5 cm of the
pylorus.
Duodenal ulcers usually occur in the first centimeter or two distal to the pylorus on the
anterior or posterior wall rather that laterally (kissing ulcers).
Classic peptic ulcer is small (about 1 cm in the duodenum; 1 to 2,5 cm in the stomach),
round-to-oval. It is characteristically “punched out”, with sharply defined margins, and has
overhanging mucosa producing a flashlike appearance. Its edges are not raised, and the
mucosal folds covering on the ulcer are distinct to its edge. Frequently it has a terraced
structure.
Malignant gastric ulcers are generally bowel shaped, with margins that are usually sloped
and generally without overhanging mucosa. The edges are raised and indurated, and
nodular mucosal or submucosal thickening interrupts the mucosal folds toward the crater.
Microscopically:
1. The bed of the ulcer is covered by fibrinous exudate containing fragmented leukocytes.
2. Fibrinoid necrosis.
3. Granulation tissue with plasma cell and lymphocytic infiltration.
4. Fibrous tissue.
The principal complications of peptic ulcer
112
I. Ulcerative-destructive:
Perforation. Anterior duodenal ulcers may perforate into the free peritoneal cavity, with
resultant peritonitis. The peritonitis from perforated peptic ulcer is initially a chemical
inflammation, but bacterial contamination soon follows. After successful surgical treatment
of the perforation, there is a risk that infected material lodged between the liver and
diaphragm may become sealed off by fibrinous exudate and cause an abscess that may later
infect the pleura.
Penetration. Extension of the inflammation to the serous coat may result in adhesion to
the adjacent organs. Perforating posterior ulcers more often penetrate the pancreas,
producing intractable pain. Posterior perforation also may occur into the lesser peritoneal
sac, leading to localized peritonitis. The omentum or adhesions to adjacent organs may also
serve to localize peritoneal inflammation.
Hemorrhage. Both gastric and duodenal ulcers are subject to massive hemorrhage.
Duodenal ulcers are especially prone to perforation. Any ulcer, but especially those located
posterior, may bleed in smaller amounts, producing melena or evidence of occult blood in
the stool. It may be abundant and give rise to “coffee-grounds” vomit. Sometimes a major
artery may be eroded and a large, even fatal, hemorrhage takes place.
II. Ulcerative- cicatricial (obstruction or healing and scarring).
Pyloric obstruction may be a complication of an ulcer, gastric or duodenal, situated near the
pylorus. It usually results from a combination of cicatricial narrowing and spasm.
Scarring in the duodenum may lead to serious stricture (pyloric stenosis). The stomach
becomes greatly dilated and hypertrophied and lead to chronic vomiting with alkalosis and
malnutration.
III. Malignization.
The development of carcinoma has been reffered to as one of the complications of peptic ulcer.
It seems probable that carcinoma can develop in a preexisting ulcer, but it is equally probable that it is
a rare event. It is extremely difficult to establish the occurrence of such a sequence of events in any
particular case.
IV. Inflammatory (gastritis, perigastritis, duodenitis, periduodenitis).
V. Mixed.
Appendicitis
Appendicitis results in severe acute or chronic inflammation of the vermiform appendix.
Acute appendicitis
Acute appendicitis is the most common acute abdominal condition requiring surgery.
Acute appendicitis is uncommon at the extremes of age and it is most frequently seen in
elder children and young adults.
The most important factor in its pathogenesis is obstruction of the lumen, with the most
frequent cause being a fecalith, a molded mass of inspissated fecal material that may
develop rock-hard consistency.
Other causes of obstruction are scars representing a residuum of previous attacks of
appendicitis, tumors, external bands, and adhesions, rarely masses of parasites, foreign
bodies, and possibly spasm of the muscle at the base of the appendix.
The immediate cause of acute appendicitis is bacterial infection from the intestinal lumen,
though bacterial invasion from the bloodstream in systemic disease is possible.
The appendix may be involved in diseases primarily affecting other portions of the
gastrointestinal tract, such as Crohn’s disease, typhoid fever, and amebiasis, and in certain
systemic diseases (such as measles).
Clinical-morphological classification of acute appendicitis
1. Simple appendicitis is characterized by hyperemia; small hemorrhages and primary affect
including small foci leucocytes.
2. Superficial appendicitis is characterized by focus of suppurative inflammation in mucosa
and edema. Serous membrane is dim.
3. Destructive forms:
Flegmonous appendicitis occurs the diffuse infiltration of leucocytes in wall of
appendix. Gross appearance: appendix is increased, swollen; tense and markedly congested
and covered by fibrinous exudate.
113
Flegmonous-ulcerative appendicitis is characterized by flegmonous inflammation
with necrosis and ulceration in mucosa.
Apostematous appendicitis the formation of small abscesses occurs. The primary
inflammatory lesion may increase in intensity and lead to a small abscess in the wall, and
this may perforate.
Gangrenous appendicitis occurs large areas of necrosis, the immediate antecedent of
rupture and may has two causes:
a) Thrombosis and thromboembolism of mesentery artery (primary gangrene of
appendix) due to obstraction of the lumen by fecoliths.
b) Thrombosis due to development of periappendicitis (secondary gangrenous
appendicitis).
The complications of acute appendicitis
1. Necrosis of appendix wall (gangrenous appendicitis), leading to perforation, with subsequent
generalized peritonitis.
2. Involvement of adjacent bowel loops, causing perforation of small bowel.
3. The omentum may become adherent, localizing the peritonitis to the right iliac fossa. Fibrosis
and continued inflammation cause development of a mass in the right iliac fossa. This may
resolve with scarring, may form an abscess that drains to the surface, or may rupture, with
development of generalized peritonitis.
4. Empyema of appendix due to obstruction of proximal parts.
5. Spread of infection by portal vein branches may propagate to the liver; this was formerly an
important cause of portal pyemic abscesses in the liver.
Chronic appendicitis
Chronic appendicitis is characterized by sclerosis and atrophy, lipomatosis and diffuse
infiltration by lymphocytes and hystiocytes.
Obliteration of part or all of the appendiceal lumen by a mixture of fibrous tissue,
lymphocytes, lymphoid follicles, and nerve bundles is common.
In the fibrosis causes complete of the lumen, continued mucous secretion might result in
cystic dilatation – mucocele.
Such a cyst may rupture, giving rise to myxoma peritonei: the mucus-secreting epithelium
is spilled into the peritoneal cavity and loculations of mucin and adhesions result.
Surgically removed appendix may be histologically normal (false-positive clinical diagnosis). If
the appendix is normal, but clinical cymptomes took place is called “false appendicitis”. It may be
due to mimicking acute appendicitis some diseases: salpingitis, ectopic pregnancy, Meckel’s
diverticulitis, peptic ulcer, and pain cause by trivial pelvic bleeding at the time ovulation.
DISEASES OF THE LIVER
There are various diseases of the liver.
In some instances, the disease is primary to the liver, as in viral hepatitis and hepatocellular
carcinoma.
More often the hepatic involvement is secondary, often to some of the most often diseases
in humans, such as cardiac decompensation, disseminated cancer, alcoholism, and
extrahepatic infections.
Some general aspects of liver disease are reviewed.
Morphologic patterns of hepatic injury
The liver is an inherently simple organ, with a limited repertoire of responses to injurious
events. Regardless of cause, five general reactions may occur.
I. Necrosis. Virtually any significant insult to the liver may cause hepatocyte necrosis.
In ischemic necrosis, poorly stained mummified hepatocytes remain (coagulative necrosis).
Necrosis of scattered hepatosytes, clumps, or an entire lobule. Isolated necrotic hepatocytes
appear as eosinophilic rounded - up, shrunken cells and are called Councilman Bodies or
apoptotic bodies).
Alternatively, hepatocytes may osmotically swell and rupture so-called hydropic
degeneration.
114
Necrosis may be limited to scattered cells within the hepatic lobules (focal necrosis) or
involve particular regions of the lobule (zonal necrosis), entire lobules (submassive
necrosis), or the whole liver (massive necrosis).
a) Focal necrosis is most characteristic of microbial infections, particularly smoldering
forms of viral hepatitis.
b) Centrilobular necrosis is characteristic of ischemic injury and many drug and toxic
chemical reaction.
c) Midzonal necrosis is a rare pattern, seen in yellow fever. Strictly periportal necrosis is
seen primarily in phosphorus poisoning and eclampsia.
d) Massive necrosis is most commonly caused by severe chemical and drug toxicity or
viral hepatitis.
In other conditions, such as typhoid fever, tularemia, brucellosis, and herpes or adenovirus
infection, expanding regions of the parenchyma are destroyed (geographic necrosis). With
disseminated candidal or bacterial infection, macroscopic abscesses may occur.
II. Degeneration.
Short of outright necrosis, hepatocytes may take on a swollen, edematous appearance
(ballooning degeneration) with irregularly clumped cytoplasm and large, clear spaces.
Alternatively, retained biliary material may impart a diffuse foamy swollen appearance to
the hepatocyte (cholestasis).
Accumulation of specific substances in viable hepatocytes, such as iron, copper, and viral
particles, may be of particular diagnostic value.
III. Inflammation. Inflammation is defined as the influx of acute or chronic inflammatory cells into
the liver and is termed hepatitis.
Although inflammation may be secondary to hepatocellular necrosis, lymphocytic attack of
viable antigen-expressing liver cells is a common cause of liver damage.
Inflammatory cells may be limited to the site of entry (portal tracts) or spill over into the
parenchyma.
In the case of focal hepatocyte necrosis, scavenger macrophages quickly generate scattered
clumps of inflammatory cells in an otherwise innocuous parenchyma.
Foreign bodies, organisms, and a variety of drugs may incite a granulomatous reaction.
IV. Regeneration.
The liver has enormous reserve, and regeneration occurs in all but the most fulminant
diseases. Regeneration is signified by thickening of the hepatocyte cords (the result of
hepatocyte proliferation) and some disorganization of the parenchymal structure.
When massive hepatocellular necrosis occurs and leaves the connective tissue framework
intact, almost perfect restitution can occur.
V. Fibrosis.
Fibrous tissue is formed in response to inflammation or direct toxic insult to the liver.
Deposition of collagen has lasting consequences on hepatic patterns of blood flow and
perfusion of hepatocytes.
In the initial stages, fibrosis may develop around portal tracts or the central vein or may be
deposited directly within the space of Disse.
With continuing fibrosis, the liver is subdivided into nodules of regenerating hepatocytes
surrounded by scar tissue, termed cirrhosis.
Classification of the liver diseases
1.
2.
3.
4.
Hepatosis (when degeneration and necrosis inflammation in the hepatocytes prevail).
Hepatitis (when inflammation in the liver prevails).
Cirrhosis (when disregeneration is observed).
Hepatic tumors.
Hepatosis
The term hepatosis is used to describe degeneration and necrosis in the liver caused by
infectious, toxic, circulatory or traumatic agents.
Hepatosis may be inherited and acquired. Inherited hepatosis develops in storage diseases
or enzymopathy. Acquired hepatosis may be acute and chronic.
The massive necrosis is the most common acute hepatosis.
The steatosis (fat hepatosis) is the most common chronic one.
115
Massive necrosis (toxic degeneration of the liver)
Massive necrosis (toxic degeneration of the liver) is acute (rarely chronic) disease
characterized by massive necrosis of the hepatocytes with development of the hepatic insufficiency.
Etiology. It is most commonly caused by viral hepatitis, drug or mushroom toxicity.
Morphology
There are 2 stages in this hepatosis.
1. Stage of yellow degeneration, when liver becomes enlarged, dense and yellow. Then it size
increases; it consistency becomes flabby; capsule is shrunken. The cut surface is grey.
Microscopically fat degeneration, necrosis and autolysis of hepatocytes are observed.
2. Stage of red degeneration is characterized by progressive reduction of liver size and mass.
Macroscopically the liver is red due to necrosis end autolysis of hepatocytes with appearance
of plethoric blood vessels. Jaundice, hyperplasia of lymph nodes and spleen, numerous
hemorrhages in the skin and mucous, necrosis of the renal epithelium, degenerative and
necrotic changes in pancreas, myocardial, CNS are observed in the patients with massive
necrosis of the liver.
Steatosis
It is a chronic disease, which is characterized by increase of fat amount in the cytoplasm of
the hepatocytes.
Etiology of steatosis is similar to massive necrosis of the liver. But in this pathologic agent
has less toxicity and, as a rule, compensatory and adaptive processes are higher.
Macroscopically the liver is enlarged, flabby. Fat drops are seen on the incision. The color is
yellow. This is called “goose” liver.
Microscopically - dust-like, small and large drop in the liver cells are observed.
Viral hepatitis
Viral hepatitis is reserved for infection of the liver caused by a small (but growing) group of
viruses having a particular affinity for the liver: Hepatitis A Virus (HAV), Hepatitis B Virus (HBV),
Hepatitis C Virus (HCV), Hepatitis D Virus (HDV), and Hepatitis E Virus (HEV).
I. Hepatitis A virus (HAV) causing a fecally spread self-limiting disease. Hepatitis A is responsible
for 20-25% of clinical hepatitis in the developing countries of the world. The disease occurs in
epidemic form as well as sporadically. The spread is related to close personal contact such as in
overcrowding, poor hygiene and sanitation. An incubation period carries on 15-45 days. HAV does
not cause chronic hepatitis. The fatality rate associated with HAV is about 0.1%.
II. Hepatitis B virus (HBV), causing a parenterally transmitted disease that may become chronic. An
incubation period carries on 4 to 26 weeks. HBV can produce:
1. Acute hepatitis.
2. Chronic nonprogressive hepatitis.
3. Progressive chronic disease ending in cirrhosis.
4. Fulminant hepatitis with massive liver necrosis.
5. An asymptomatic carrier state with or without progressive disease.
Further more HBV plays an important role in the development of hepatocellular carcinoma.
Transfusion, blood products, dialysis, needle-stick accidents among health care workers, intravenous
drug abuse, and homosexual activity constitute primary risk categories for HBV.
III. Hepatitis C virus (HCV), also tenned non-A, non-B (NANB) hepatitis virus involved chiefly in
transfusion-related hepatitis. HCV has a high rate of progression to chronic disease and eventual
cirrhosis, exceeding 50%.
IV. Delta hepatitis virus (HDV) is acute coinfection by exposure to serum containing both HDV and
HBV. Hepatitis may be mild to fulminant, with fulminant disease somewhat more likely than with
HBV alone; chronicity rarely develops.
Morphological patterns of Acute Viral Hepatitis
Any one of the hepatotropic viruses can cause acute viral hepatitis. Whatever the agent, the
disease is more or less the same and can be divvied into four phases:
1. An incubation period.
2. A symptomatic preicteric phase.
116
3. A symptomatic icteric phase.
4. Convalescence.
The morphologic changes in acute viral hepatitis are virtually the same regardless of the
causative agent and can be mimicked by drug reactions.
Grossly, the liver is slightly enlarged: more or less green depending on the phase of the
acute disease and the degree of jaundice.
Histologically the major findings are:
Hepatocellular injury:
Necrosis of scattered hepatocytes, clumps, or an entire lobule.
Isolated liver cells or small cell clusters appear as eosinophilic rounded-up cells
(apoptotic bodies, Councilman’s bodies).
Degenerated hepatocytes may also appear ballooned. Fatty change is unusual except with
HCV.
Macrophages may phagocytize the necrotic hepatocytes; and may accumulate clumps of
lymphocytes and macrophages.
Confluent necrosis may lead to bridging necrosis connecting portal-to-portal, central-tocentral, or portal-to-central regions of adjacent lobules, signifying a more severe form of
acute hepatitis.
Inflammation is a characteristic, usually prominent feature of acute hepatitis.
The portal tracts are usually infiltrated with a mixture of inflammatory cells; this infiltrate
consists of lymphocytes with a touch of leucocytes and may spill over into the parenchyma,
particularly where adjacent hepatocytes have undergone necrosis.
Reactive changes in Kupffer’s cells.
Kupffer cells and sinusoidal lining cells undergo hypertrophy and hyperplasia and are often
laden with lipofuscin pigment owing to phagocytosis of hepatocellular debris.
Cholestasis is biliary stasis.
An inconstant finding is bile stasis within the lobule. The bile duct epithelium may
proliferate, particularly in cases of HCV hepatitis, forming poorly defined ductular
structures (cholangioles).
Regeneration.
In the recovery phase of acute hepatitis, the lobule remains somewhat disorganized because
hepatocytes can proliferate faster than normal cord-sinusoid-cord relationships can be
established.
Regenerating hepatocytes lack uniformity in size and are pale, the result of diminished
numbers of cytoplasmic organelles.
Double and triple nuclei in regenerating cells are commonly observed. Residual clumps of
inflammatory cells may persist for some time.
Lobular disarray results from the cellular swelling (ballooning), necrosis, and regeneration
of cells producing compression of the vascular sinusoids and loss of the normal, more or
less radial array. Disruption of lobular architecture by necrosis is called lobular disarry.
Morphological patterns of Chronic Viral Hepatitis
Symptomatic, biochemical, or serologic evidence of continuing or relapsing hepatic disease
for more than 6 months, optimally with histologically documented inflammation and
necrosis, is taken to mean chronic hepatitis.
Although the hepatitis viruses are responsible for most cases of chronic hepatitis, there are
many other etiologies: Wilson’s disease, alpha-1-antitrypsin deficiency, chronic alcoholism,
drugs (isoniazid, alpha-methyldopa, methotrexate), and autoimmunity.
Since 1968, chronic hepatitis has been classified according to the extent of inflammation:
1. Chronic persistent hepatitis, in which inflammation is confined to the portal tracts.
2. Chronic active hepatitis, in which portal tract inflammation spills into the
parenchyma and surrounds regions of necrotic hepatocytes.
3. Chronic lobular hepatitis, in which persistent inflammation is confined to the
lobule.
It is now apparent that the primary determinant of disease progression, and therefore
prognosis, is the etiologic form of hepatitis. Therefore, although histologic information may
provide information helpful for patient management, classification of chronic hepatitis
strictly by histologic criteria is obsolete and should not be used. This is particularly
117
important because therapy that is effective for one cause of chronic hepatitis may be
ineffective, or potentially detrimental, in other forms of the disease.
The likelihood of chronic hepatitis following acute viral infection can be summarized:
1. HAV: Extremely rare.
2. HBV: Develops in more than 90% of infected neonates and 5% of infected adults, of
whom one-fourth progress to cirrhosis.
3. HCV: Develops in more than 50% of infected patients, half of whom progresses to
cirrhosis.
4. HDV: Rare in acute HDV/HBV coinfection; a more severe chronic hepatitis is the
most frequent outcome of HDV superinfection.
5. HEV: Does not produce chronic hepatitis.
Chronic hepatitis with HBV, and apparently with HCV, contributes significantly to the
development of primary hepatocellular carcinoma.
Morphology
The morphology of chronic hepatitis ranges from exceedingly mild to severe, to eventual
cirrhosis.
The diagnosis of chronic persistent hepatitis is confirmed by needle biopsy of the liver,
which is invaluable in distinguishing it from more serious form of chronic active hepatitis.
Microscopically:
There is portal triad characterized by expansion of the portal tract by mononuclear
inflammatory cells, consisting of lymphocytes, macrophages, occasional plasma cells, and
an occasional rare neutrophiles or eosinophiles.
The lobular architecture of hepatic parenchyma is usually preserved.
There is absence of piecemeal necrosis.
Chronic active (aggressive) hepatitis is defined as a progressive form of chronic
necrotising and fibrosing disease involving portal tracts as well as hepatic parenchyma.
Microscopically:
The histologic hallmark of progressive disease is piecemeal necrosis, where by the chronic
inflammatory infiltrate spills out from portal tracts into adjacent parenchyma, with
associated necrosis of hepatocytes in the limiting plate.
There may be formation of lymphoid follicles.
There may be lobular inflammation with focal necrosis of hepatocytes.
As with acute hepatitis, bridging necrosis may connect adjacent portal-portal, centralcentral, and portal-central zones.
Although piecemeal and bridging necrosis do not imply inevitable progression of disease,
continued loss of hepatocytes results in fibrous septum formation, which, accompanied by
hepatocyte regeneration, results in cirrhosis.
The aforementioned features are common to all forms of chronic hepatitis (viral or otherwise).
In patients with chronic HCV hepatitis, lymphoid aggregates in portal tracts and mild fatty change are
seen in about 50% of cases, and bile duct damage is seen in more than 90%. Conversely, “groundglass” hepatocytes are sometimes present in chronic HBV hepatitis. Despite use of immunohistochemical techniques, it is frequently impossible to identify the etiology of chronic hepatitis on
tissue samples, so great reliance must be placed on clinical, virologic, and serologic observations.
The clinical features of chronic hepatitis
The clinical features of chronic hepatitis are extremely variable and are not predictive of
outcome.
In some patients, the only signs of chronic disease are persistent elevations of serum
transaminases, hence the facetious designation “transaminitis”.
The most common symptom is fatigue; less common symptoms are malaise, loss of
appetite, and occasional bouts of mild jaundice.
Physical findings are few, if any, the most common being spider angiomas, palmar
erythema, mild hepatomegaly, hepatic tenderness, and mild splenomegaly.
Laboratory studies may reveal prolongation of the prothrombin time and, in some
instances, hyperglobulinemia, hyperbilirubinemia, and mild elevations in alkaline
phosphatase.
118
Occasionally in cases of HBV, and rarely in HCV, immune-complex diseases may develop
secondary to the presence of circulating antibody-antigen complexes, in the form of
vasculitis (subcutaneous or visceral, i.e., polyarteritis nodosa) or glomerulonephritis.
The major causes of death are hepatic insuffisiency and hepatic encephalopathy or massive
hemorrhage from esophageal varicose and, in those with long-standing HBV (particularly
neonatal) or a HCV infection, hepatocellular carcinoma.
Cirrhosis of Liver
Cirrhosis is the final stage of liver disease and is defined by three characteristics:
1. Fibrosis is present in the form of delicate bands or broad scars replacing multiple
adjacent lobules.
2. The parenchymal architecture of the liver is divided by interconnecting fibrous scars.
3. Parenchymal nodules are created by regeneration of hepatocytes. The nodules may
vary from micronodules (less than 3 mm in diameter) to macronodules (3 mm to
several centimeters in diameter).
Several features should be understood:
1. The parenchymal injury and consequent fibrosis are diffuse, extending throughout the
liver; focal injury with scarring does not constitute cirrhosis.
2. Nodularity is requisite for the diagnosis and reflects the balance between regenerative
activity and constrictive scarring.
3. The fibrosis, once developed, is generally irreversible; some regression has been
observed in humans with treated schistosomiasis and hemochromatosis.
4. Vascular architecture is recognized by parenchymal damage and scarring, with
formation of abnormal interconnections between vascular inflow and hepatic vein
outflow.
Classification
Morphological types of cirrhosis
1. Micronodular (the nodules are usually regular and small, less than 3 mm in diameter).
2. Macronodular (the nodules are of variable size and are generally large than 3 mm in diameter).
3. Mixed (some part of the liver show micronodular appearance while other parts show
macronodular pattern).
Each of these forms may have an active and inactive form:
- Hepatocellular necrosis and inflammatory reaction, a process that closely resembles
chronic active hepatosis characterizes an active form.
- An inactive form, vise verse, has no evidence of continuing hepatocellular necrosis
and has sharply-defined nodules of surviving hepatic parenchyma without any
significant inflammation.
Etiologic types of cirrhosis
1. Infectious (often viral).
2. Toxic and toxic-allergic (Alcoholic cirrhosis, the most common, 60-70%; allergen,
drugs, etc.).
3. Biliary cirrhosis (5-10%).
4. Metabolic-alimentary (Cirrhosis in Wilson’s disease, Cirrhosis in α-l antitrypsin
deficiency, Pigment cirrhosis in hemochromatosis (5%), etc.).
5. Cardiac cirrhosis.
6. Cryptogenic cirrhosis (10-15%).
Types according to morphogenesis
1. Postnecrotic cirrhosis.
2. Portal (septical) cirrhosis.
3. Mixed cirrhosis.
Morphological patterns of cirrhosis
119
Morphological changes of all types of cirrhosis are similar.
Macroscopically the liver is small, having distorted shape with irregular and coarse scars
and nodules of varying size. The cut surface shows scars and nodules varying in diameter
from 3 mm to a few centimetres.
Microscopically, the features are following:
Abnormal lobular architecture can be identified and central veins are hard to find.
The fibrous septa dividing the variable-sized nodules are generally thick.
Active liver cell necrosis is observed. Fibrous septa contain prominent mononuclear
inflammatory cell infiltrate even with follicles. Often there is extensive proliferation of
bile ductules derived from collapsed liver lobules.
Liver cells vary considerably in size and multiple large nuclei are common in
regenerative nodules. Fatty degeneration may be present.
Postnecrotic cirrhosis
This pattern of cirrhosis is characterized by irregularly sized nodules separated by variable
but mostly broad scars.
The most common known cause is previous viral infection; in about 20 to 25% of cases it
evolves from chronic HBV infection; the contribution of chronic HCV may be even greater.
In a small number of instances, there is a well-documented history of acute liver damage
caused by some hepatotoxin, such as phosphorus; carbon tetrachloride; mushroom
poisoning; or a drug such as acetaminophen, oxyphenisatin, or alpha-methyldopa.
Undoubtedly some cases represent end-stage alcoholic cirrhosis, readily misinterpreted as
postnecrotic cirrhosis in the absence of a history of chronic alcoholism.
After all these possibilities have been excluded, there remains a large residual of uncertain
origin.
A single attack of massive hepatic necrosis only infrequently gives rise to postnecrotic
cirrhosis because either it is fatal, or regeneration of the liver cells permits survival with
little or no residual scarring.
Morphology
Typically some time after an acute event or following years of chronic hepatitis, the liver
exhibits nodules of varying size (some several centimeters in diameter) and broad bands or
areas of depressed scarring.
Severe collapse may leave a shrunken liver less than 1 kg in size.
Microscopically tubular architecture may be completely lost in the developing nodules and
scar.
Alternatively, progressive chronic hepatitis of any etiology inexorably transforms a more
normalized liver into a patchwork of variably sized nodules alternating with broad septal
scars.
Eventually active liver cell necrosis becomes inconspicuous.
Residua of portal tracts may be evident; bile stasis is variable.
Ultimately the diagnosis rests on excluding other bases for cirrhosis.
Biliary cirrhosis
Biliary cirrhosis is defined as a chronic disorder characterized by clinical, biochemical and
morphological features of long-continued cholestasis of extrahepatic or intrahepatic origin. There is
primary and secondary biliary cirrhosis.
Primary biliary cirrhosis (PBC)
Primary biliary cirrhosis (PBC) is autoimmune disorder focused on intralobular bile ducts
and holangioles, this disease causes chronic inflammation of intrahepatic bile ducts, leading
to their destruction and, in time, cirrhosis.
The primary feature of this disease is a nonsuppurative, granulomatous destruction of
medium-sized intrahepatic bile ducts; cirrhosis appears only late in the course.
This is primarily a disease of middle-aged women, with a female-to-male predominance in
excess of 6:1. Age of onset is between 20 and 80 years, with the peak incidence between 40
and 50 years.
The onset is insidious, usually presenting with pruritus. Jaundice develops late in the
course.
120
Hepatomegaly is typical. Xanthomas and xanthelasmas arise as a result of cholesterol
retention. Stigmata of chronic liver disease are late features.
The disease may be asymptomatic for years, running its course over two or more decades.
PBC is the prototype of all conditions leading to small-duct biliary fibrosis and cirrhosis.
Historically, four histologic stages have described:
1) The duct lesion (granulomatous destruction of interlobular bile ducts). There is
random, focal destruction of interlobular and septal bile ducts by granulomatous
inflammation, named the florid duct lesion. Affected portal tracts exhibit a dense
infiltrate of lymphocytes (including lymphoid follicle formation), histiocytes, plasma
cells, and a few eosinophils. Parenchymal holestasis may be present.
2) Ductular proliferation with periportal hepatitis. With more global hepatic
involvement, normal interlobular bile ducts become infrequent, and secondary
obstructive changes develop, similar to those seen in extrahepatic obstruction. Mallory
bodies (alcoholic hyaline) may be present in hepatocytes adjacent to portal tracts.
Initially portal tract inflammation may be marked and spill over into the parenchyma,
causing destruction of adjacent hepatocytes (piecemeal necrosis).
3) Fibrosis. With time, inflammation decreases; granulomas and duct lesions become
infrequent and are replaced by fibrous septa. Bile ducts are reduced; holestasis is
prominent.
4) Cirrhosis. Hepatocyte loss, fibrosis, and nodular regeneration lead to the gradual
development of true cirrhosis. Macroscopically the liver does not at first appear
abnormal, but as the disease progresses, bile stasis stains the liver green. The capsule
remains smooth and glistening until a fine granularity appears, culminating in a well
developed, uniform micronodularity. Liver weight is at first normal to increased
(owing to inflammation); ultimately liver weight is slightly decreased. In most cases,
the end-stage picture may be difficult to distinguish from secondary biliary cirrhosis or
the cirrhosis that follows chronic active hepatitis.
Secondary biliary cirrhosis
Develops with prolonged extrahepatic biliary tract obstruction.
The most common cause of obstruction is an impacted gallstone in the common bile duct;
other conditions include biliary atresia, malignancies of the biliary tree and head of the
pancreas, and strictures resulting from previous surgical procedures.
Retained bile leads to inflammation initiating periportal fibrosis and eventual cirrhosis.
Secondary bacterial infection ("ascending cholangitis") may contribute to the damage;
enteric organisms such as coliforms and enterococci are common culprits.
Macroscopically, the liver is of yellow-green color and is accompanied by marked icteric
discoloration of body tissues and fluids. On cut surface, the liver is hard, with a finely
granular appearance.
Microscopically:
Large and small bile ducts are distended and frequently contain inspissated bile.
Portal tracts are interconnected by inflamed fibrous septa and appear edematous; there
is frequently a narrow zone of edema and ductular proliferation at the junction of
parenchyma and septa.
Cholestatic features may be severe, with cytoplasmic and canalicular accumulation of
bile, extensive feathery degeneration of hepatocytes, and the formation of bile lakes
(see earlier discussion of cholestasis).
Once the regenerative nodules of cirrhosis have formed, however, bile stasis may
become less conspicuous.
Ascending bacterial infection incites a supervening robust neutrophilic infiltration of
bile ducts and cholangitic abscesses.
Cardiac cirrhosis
Cardiac cirrhosis is uncommon complication of severe right-sided congestive heart
failure of long-standing duration.
121
The common causes culminating in cardiac cirrhosis are “cor pulmonale”, tricuspid
insufficiency or constrictive pericarditis.
Microscopically, the hepatic sinusoids are dilated and congested with hemorrhagic necrosis
of centrolobular hepatocytes.
Then fibrous strands radiating from the central veins are observed.
Alcoholic liver disease
Alcohol abuse constitutes the major form of liver disease in many countries.
Chronic alcohol consumption has a variety of adverse effects. Of greatest impact, however,
there are three distinctive, albeit overlapping, forms of liver disease:
1. Hepatic steatosis.
2. Alcoholic hepatitis.
3. Cirrhosis referred to as alcoholic liver disease.
Hepatic steatosis
Following even moderate intake of alcohol, small (microvesicular) lipid droplets accumulate
in hepatocytes.
With chronic intake of alcohol, lipid accumulates to the point of creating large clear
macrovesicular spaces, compressing and displacing the nucleus to the periphery of the
hepatocyte.
This transformation is initially centrolobular, but in severe cases, it may involve the entire
lobule.
The liver is often grossly enlarged, up to 4 to 6 kg, and is a soft, yellow, greasy organ.
Although there is little or no fibrosis at the outset, with continued alcohol abuse, fibrous
tissue develops around the central veins and extends into the adjacent sinusoids.
Up to the time that fibrosis appears, the fatty change is completely reversible if there is
further abstention from alcohol.
Alcoholic hepatitis
Alcoholic hepatitis exhibits the following:
Liver cell necrosis, single or scattered foci of cells undergo swelling (ballooning) and
necrosis, more frequently in the centrolobular regions of the lobule.
Mallory bodies (alcoholic hyaline), scattered hepatocytes accumulate tangled skeins of
cytokeratin intermediate filaments and other proteins, visible as eosinophilic cytoplasmic
inclusions.
Neutrophilic reaction. Neutrophils permeate the lobule and accumulate around
degenerating liver cells, particularly those having Mallory bodies. Lymphocytes and
macrophages also enter portal tracts and spill into the lobule. Potentially reversible, this
lesion may smaller on long after cessation of alcohol intake.
Fibrosis. Alcoholic hepatitis is almost always accompanied by a sinusoidal and perivenular
fibrosis; occasionally periportal fibrosis may predominate, particularly with repeated bouts
of heavy alcohol intake. Fat may be present or entirely absent. Deranged iron processing in
the alcoholic typically leads to a modest accumulation of hemosiderin in hepatocytes and
Kupffer’s cells. The outcome is unpredictable. The condition may resolve in the absence of
further alcohol exposure or may lead to cirrhosis, but there is significant risk of death with
each bout.
Alcoholic cirrhosis
The final and irreversible form of alcoholic liver disease usually evolves slowly and
insidiously.
At first the cirrhotic liver is yellow-tan, fatty, and enlarged, usually weighing more than 2
kg.
Over the span of years, it is transformed into a brown, shrunken, nonfatty organ, and
sometimes less than 1 kg in weight.
Cirrhosis may develop within 1 to 2 years in the setting of alcoholic hepatitis.
Initially the developing fibrous septa are delicate and extend from central vein to portal
regions as well as from portal tract to portal tract.
Regenerative activity of the entrapped parenchymal acini generates fairly uniformly sized
“micronodules”.
122
With time, the nodularity becomes more prominent; scattered nodules may become quite
large, and occasionally nodules more than 2 cm in diameter may develop.
As fibrous septa dissect and surround nodules, the liver becomes more fibrotic, loses fat,
and shrinks progressively in size.
Parenchymal islands are engulfed by ever wider bands of fibrous tissue, and the liver is
converted into a mixed micronodular and macronodular pattern.
Further ischemic necrosis and fibrous obliteration of nodules eventually create broad
expanses of tough, pate scar tissue, leaving residual parenchymal nodules that protrude like
“hobnails” from the surface of the liver (“Laennec’s cirrhosis”).
By microscopy, the septa contain variable amounts of scattered lymphocytes and some
reactive bile duct proliferation. Bile stasis often develops; Mallory bodies are only rarely
evident at this stage.
Thus, end-stage alcoholic cirrhosis comes to resemble, both macroscopically and
microscopically, postnecrotic cirrhosis.
Complications of cirrhosis
Complications of cirrhosis are subdivided into 2 groups: hepatic and non-hepatic
I. Hepatic complication:
Progressive hepatic insufficiency.
Development of hepatocellular carcinoma.
Steatorrhea due to reduced hepatic bile secretion.
Gall stones usually of pigment type, are seen twice more frequently in patients with
cirrhosis than in general population.
II. Non-hepatic complication:
1. Portal hypertension (increased resistance to portal flow) and its effects such as
Ascites.
The formation of portosystemic venous shunts through variceal chanells in the esophagus,
rectum, and periumbilical abdominal wall.
Congestive splenomegaly.
Hepatic ehcephalopathy.
2. Chronic relapsing pancreatitis, especially in alcoholic liver disease.
3. Infections are more frequent in patients with cirrhosis due to impaired phagocytic activity of
reticuloendothelial system.
4. Hematological derangements such as bleeding disorders and anemia due to impaired hepatic
synthesis of coagulation factors and hypoalbuminemia are present.
5. Cardiovascular complications such as atherosclerosis of coronaries and aorta and myocardial
infarction are more frequent in these patients.
6. Hypertrophic osteoarthropathy.
7. Endocrine disorders such as gynecomastia, testicular atrophy and impotence, whereas in cirrhotic
women amenorrhoe is a frequent abnormality.
8. Hepatorenal syndrome leading to renal failure may occur in late stages of cirrhosis.
Causes of death
1. Hepatic coma.
2. Massive gastrointestinal hemorrhage from esophageal varice.
3. Intercurrent infections.
4. Hepatorenal syndrome.
5. Development of hepatocellular carcinoma.
Cholelitiasis (Gallstones)
Gallstones are formed from constituents of the bile (viz. cholesterol, bile pigments and
calcium salts) along with other organic components.
Accordingly, the gallstones commonly contain cholesterol, bile pigment and calcium salts in
varying proportions.
They are usually formed in the gall bladder, but sometimes may develop within extrahepatic
biliary passages, and rarely in the larger intrahepatic bile duct.
The incidence of gallstones varies markedly in different geographic areas, age, sex, diet and
various other risk factors.
The mechanism of cholesterol gallstone formation or lithogenesis is determined by 3 major
factors:
123
1) Namely supersaturation of bile with cholesterol.
2) Cholesterol nucleation.
3) The hyperfunction of gallbladder.
Types of gallstones. As stated before, gallstones contain cholesterol, bile pigment and
calcium carbonate, either in pure form or in various combinations. Gallstones are of 3
major types:
1) Pure gallstones
2) Mixed gallstones
3) Combined gallstones.
In general, gallstones are formed most frequently in the gall bladder but may occur in
exlrahepatic as well as intrahepatic biliary passages.
Numerous complications develop in cholelithiasis. They are cholecystitis,
choledocholithiasis, mucocele or hydrops of the gallbladder, biliary fistula, gallstone ileus,
and gallbladder cancer.
Cholecyscitis
Cholecyscitis or inflammation of the gallbladder may be acute, chronic, or acute
superimposed on chronic.
Acute cholecystitis
In many ways, acute cholecystitis is similar to acute appendicitis. The condition usually
begins with obstruction, followed by infection later.
Based on the initiating mechanisms, acute cholecystitis occurs in two types of situations acute calculous and acute acalculous cholecystitis.
In majority of cases, acute cholecystitis is caused by obstruction in the neck of the
gallbladder or in the cystic duct by a gallstone. The commonest location of impaction of a
gallstone is in Hartman’s pouch. After that secondary bacterial infection, for instance E.coli
and Streptococcus facialis, supervenes.
Acute calculous cholecystitis. The remaining 10% cases of acute cholecystitis do not contain
gallstones. In such cases, a variety of causes have been assigned such as previous nonbiliary surgery, multiple injuries, bums, severe sepsis, diabetes mellitus, etc.
Morphology
Except for the presence or absence of calculi, the two forms of acute cholecystitis are
morphologically similar.
Macroscopically, the gall bladder is distended and tense. The serosal surface is coated with
fibrinous exudate with congestion and hemorrhages. The mucosa is red. The lumen is filled
with pus mixed with green bile. In calculous cholecystitis, a stone is generally impacted in
the neck or in the cystic duct. When obstruction of the cystic duct is complete, the lumen is
filled with purulent exudate and the condition is known as empyema of the gall bladder.
Microscopically, wall of the gall bladder shows marked inflammatory edema, congestion
and neutrophilic exudate. There may be frank abscesses in the wall and gangrenous
necrosis with rupture into the peritoneal cavity (gangrenous cholecystitis).
Chronic Cholecystitis
Chronic cholecystitis is the commonest type of clinical gallbladder disease.
The association of chronic cholecystitis with mixed and combined gallstones is virtually
always present.
Macroscopically, the gall bladder is generally contracted but may be normal or enlarged.
The wall of the gall bladder is thickened which on cut section is grey-white due to dense
fibrosis or may be even calcified. The mucosal folds may be intact, thickened, or flattened
and atrophied. The lumen commonly contains multiple mixed stones or a combined stone.
Microscopically, the following signs, may be observed: thickened and congested mucosa
but occasionally mucosa may be totally destroyed; penetration of the mucosa deep into the
wall of the gall bladder up td muscular layer to form Rokitansky-Aschoff sinuses; variable
degree of chronic inflammatory reaction, consisting of lymphocytes, plasma cells and
macrophages, present in the lamina propria and subserosal layer; variable degree of fibrosis
in the subserosal and subepithelial layers.
124
Pancreatitis
Pancreatitis is inflammation of the pancreas with acinic cell injury. It is classified into acute
and chronic forms both of which are two distinct entities.
Acute pancreatitis
Acute pancreatitis is an acute inflammation of the pancreas.
The severe form of the disease associated with macroscopic hemorrhages and fat necrosis in
and around the pancreas is termed acute hemorrhage pancreatitis or acute pancreatic
necrosis.
The condition occurs in adults between the age of 40 and 70 years and is commoner in
females than in males.
The onset of acute pancreatitis is sudden, occurring after a bout of alcohol or a heavy meal.
The patient presents with abdominal pain, vomiting and collapse and the condition must be
differentiated from other diseases producing acute abdomen such as acute appendicitis,
perforated peptic ulcer, and acute cholecystitis.
Etiology. The two leading causes associated with acute pancreatitis are alcoholism and
cholelithiasis, both of which are implicated in more than 80% of cases. Less common causes
of acute pancreatitis include trauma, ischemia, shock, extension of inflammation from the
adjacent tissues, blood-borne bacterial infection, viral infections, certain drugs, etc.
Morphology
The morphology of acute pancreatic necrosis stems directly from the action of activated
pancreatic enzymes that are released into the pancreatic substance.
The basic alterations are proteolytic destruction of pancreatic substance, necrosis of blood
vessels with subsequent hemorrhage, necrosis of fat, and an accompanying inflammatory
reaction.
Amorphous basophilic calcium precipitates may be visible within the necrotic focus.
Grossly, foci of pancreatic necrosis are blue-black hemorrhages and grey-white necrotic
softening alternates with sprinkled foci of yellow-white, chalky fat necrosis.
Complications
A patient of acute pancreatitis who survives may develop a variety of systemic and local
complications:
1. Systemic complications are chemical and bacterial peritonitis, endotoxic shock, and acute renal
failure.
2. Local complications are pancreatic abscess, pancreatic pseudocyst, and duodenal obstruction.
Chronic pancreatitis
Chronic pancreatitis is the progressive destruction of the pancreas due to repeated mild
and subclinical attack of acute pancreatitis.
Most patients present with recurrent attacks of severe abdominal pain at intervals of
months to years.
Weight loss and jaundice are often associated. Later manifestations include associated
diabetes mellitus and steatorrhea.
Etiology. Most cases of chronic pancreatitis are caused by the same factors as for acute
pancreatitis.
Morphology
Chronic pancreatitis is distinguished by irregularly distributed fibrosis, reduced number
and size of acini with relative sparing of the islets of Langerhans, and variable obstruction
of pancreatic ducts of all sizes.
The lesions have a macroscopic lobular distribution and may involve portions or the entire
pancreas.
A chronic inflammatory infiltrate around lobules and ducts is usually present.
The ductal epithelium may be atrophied or hyperplastic or may show squamous metaplasia.
Macroscopically, the gland is hard and exhibits foci of calcification and may developed
pancreatic calculi. These concretions vary from calculi invisible to the naked eye, to stones 1
cm to several centimetres in diameter, giving rise to the term “chronic calcifying
pancreatitis”.
125
With chronic ductal obstruction, the distribution of lesions is irregular, and the ductal
epithelium generally is less severely damaged. Protein plugs and calcified stones are rare.
Complications
Last stage of chronic pancreatitis may be complicated by diabetes mellitus, pancreatic
insufficiency with steatorrhea and malabsorption and formation of pancreatic pseudocysts.
DISEASES OF KIDNEY AND URINARY TRACT
Diseases of the kidney are characterized by the injury basic morphologic components:
glomeruli, tubules, interstitium, and blood vessels. The clinical manifestations of renal diseases can be
grouped into reasonably well-defined syndromes.
We can now turn to a brief description of the major renal syndromes:
1. Acute nephritic syndrome is a glomerular syndrome dominated by the acute inset of usually
grossly visible hematuria (red blood cells in urine), mild to moderate proteinuria, and
hypertension; it is the classic presentation of acute poststreptococcal glomerulonephritis (GN).
2. The nephrotic syndrome is characterized by heavy proteinuria, hypoalbuminuria, severe
edema, hyperlipidemia, and lipiduria.
3. Asymptomatic hematuria or proteinuria, or a combination of them, is usually manifestation
of subtle or mild glomerular abnormalities.
4. Acute renal failure is dominated by oliguria or anuria, with recent onset of azotemia. It can
result from glomerular injury, interstitial injury, or acute tubular necrosis.
5. Chronic renal failure, characterized by prolonged symptoms and signs of uremia, is the final
result of all chronic renal diseases.
6. Renal tubular defects are dominated by polyuria, nocturia, and electrilyte disorders. They
are the result of either diseases directly affecting tubular structure or defects in specific
tubular infection. The latter may be inheridited or acquired.
7. Urinary tract infection is characterized by bacteriuria and pyuria. The infection may be
symptomatic or asymptomatic, and it may affect the kidney or the bladder only.
8. Nephrolitiasis (renal stone) is manifested by renal colic, hematuria, and recurrent stone
formation
Glomerular Diseases
Glomerular injury is a major cause of renal disease and may be primary and secondary.
1. Primary glomerular diseases are characterized by primary injury of the glomeruli (acute and
chronic glomerulonephritis (GN), lipoid nephrosis, etc.).
2. In secondary glomerular diseases the kidney is one of many organs and systems damaged by
a systemic disease ( Systemic lupus erythematous, diabetes mellitus, amyloidosis, etc.)
Pathogenesis of glomerular injury
The consequences of injury at different sites within the glomerulus can be assessed when
compared with the normal physiologic role of the main cells involved, i.e. endothelial, mesangial,
visceral epithelial, and parietal epithelial cells as well as of the GBM.
There are two basic mechanisms of glomerular injury: immune and nonimmune.
Immune mechanisms
A. Antibody-mediated glomerular injury
1. Immune complex disease.
The deposition of Ag-Ab complexes in glomeruli is a major mechanism of glomerular
injury, whether they are formed “in situ” with glomerular antigens or are trapped
circulating complexes.
Immunologic mechanisms underlying glomerular injury are primarily antibody-mediated
(immune-complex disease). More recently there has been evidence to suggest that cellmediated immune reactions in the form of delayed type hypersensitivity can cause
glomerular injury.
Glomerular deposits are formed by one of the following two mechanisms:
126
Local immune complex deposits. Formation of glomerular deposits of immune
complex “in situ” occurs as a result of combination of antibodies with autologous nonbasement membrane antigens or nonglomerular antigens planted on glomeruli. Classic
experimental model of “in situ” immune complex GN is Heymann nephritis. The examples
of planted nonglomerular antigens are cationic proteins, lectins, DNA, bacterial products
(e.g. a protein of group A streptococci), viral and parasitic products and drugs.
Circulating immune complex deposits. Circulating immune complexes cause
glomerular damage under certain circumstances, e.g. their presence in high concentrations
for prolonged periods, or when they possess properties that cause their binding to
glomeruli, or when host mechanisms fail to eliminate immune complexes. The antigenantibody complexes are trapped in the glomeruli where they produce glomerular injury
after combining with complement.
Immune complex GN is observed in the following diseases:
1. Primary GN, e.g. acute diffuse proliferative GN, membranous GN,
membranoproliferative GN, IgA nephropathy and some cases of rapidly progressive
GN and focal GN.
2. Systemic diseases, e.g. glomerular disease in SLE, malaria, syphilis, hepatitis, HenochSchonlein purpura and idiopathic mixed cryoglobulinemia.
2. Anti- GBM disease.
Less than 5% cases of human GN are associated with anti-GBM antibodies. The component
of GBM acting as antigen appears to component of collagen IV of the basement membrane.
Anti-GBM disease is classically characterized by homogeneous linear deposits of anti-GBM
antibodies (mostly IgG; rarely IgA and IgM) and complement (mainly C3) along the
glomerular basement membrane.
Anti-GBM disease is characteristically exemplified by glomerular injury in Goodpasture’s
syndrome. About half to two-third of the patients with renal lesions in Goodpasture’s
syndrome have pulmonary hemorrhage mediated by cross-reacting autoantibodies against
alveolar basement membrane.
3. Alternative pathway disease.
The complement system, in particular C3, contributes to glomerular injury in the majority
of forms of GN.
The deposits in alternate pathway of the disease are characteristically electron-dense,
glomerular lesions in such cases are referred to as dense-deposit disease.
Alternate pathway disease occurs in most cases of type II membranoproliferative GN, some
patients of rapidly progressive GN, acute diffuse proliferative GN, IgA nephropathy and in
SLE.
4. Other mechanisms of antibody-mediated injury.
A few autoantibodies have been implicated in some patients of glomerulonephritis:
Anti-neutrophil cytoplasmic antibodies (ANCA). About 40% cases of rapidly
progressive GN are deficient in immunoglobulins in glomeruli and are positive for ANCA
against neutrophil cytoplasmic antigens in their circulation. ANCA causes endothelial injury
by generation of reactive oxygen radicals.
Anti-endothelial cell antibodies (AECA). Autoantibodies against endothelial antigens
have been detected in circulation are several inflammatory vasculitis and
glomerulonephritis.
B. Cell-mediated Glomerular Injury
Recent evidence suggests that cell-mediated immune reactions in the form of delayed
hypersensitivity may be involved in causing glomerular injury, particularly in cases with deficient
immunoglobulins.
C. Secondary pathogenetic mechanisms
Secondary pathogenetic mechanisms are a number of mediators of immunologic glomerular
injury, such as: neutrophils, mononuclear phagocytes, complement system, platelets, mesangial cells,
and coagulation system.
Nonimmune mechanisms
Though most forms of GN are immunologically mediated, a few examples by non-immunologic
mechanisms are found:
1. Metabolic glomerular injury, e.g. diabetic nephropathy.
2. Hemodynamic glomerular injury, e.g. systemic hypertension.
3. Deposition diseases, e.g. cryoglobulinaemia, amyloidosis.
127
4. Infectious diseases, e.g. HBV, HCV, HIV.
5. Inherited glomerular diseases, e.g. Alport’s syndrome, nail-patella syndrome.
These diseases destroy sufficient functioning nephrons. Adaptive changes in glomeruli to the
increased workload cause epithelial and endothelial injury and resulten proteinuria. The mesangial
response, involving mesangial cell proliferation and matrix deposition, and intraglomerular
coagulationcause the glomerulosclerosis.
Acute Glomerulonephritis
The first group of glomerular diseases are characterized anatomically by inflammatory
alterations in the glomeruli and clinically by a complex of findings classically reffered to as
the syndrome of acute nephritis.
Nonrenal features, such as: arterial hypertension, hypotrophy of right heart,
disproteinemia, edema, hypernitrogenemia and uremia are presence.
It is infectious-allergic or unknown etiology disease with double nonsuppurative
glomerulitis.
The nephritic patient usually presents with hematuria, red cell casts in the urine, azotemia,
oliguria, and mild to moderate hypertension.
The patient also commonly has proteinuria and edema, but these are not as several those
encountered in the nephrotic syndrome. The acute nephritic syndrome may occur in such
multisystem diseases as SLE and polyarteritis nodosa. Typically, however, it is
characteristic of acute proliferative GN and is an important component of crescentic GN.
Principles of glomeluronephritis classification
Gomerulonephritis may be primary or secondary.
According to the etiology it may be bacterial, viral, unclear.
According to the pathogenesis there are 2 types of glomeluronephritis: -immunoassociated and non-immunoassociated.
According to the course GN may be classified into acute, sub-acute, chronic.
According to the histological pattern of damage seen on renal biopsy; hence knowledge of
this aspect of histopathology is needed to understand disease. In morphological
classification, topography, character, propagation of pathological process are accounted:
1. According to topography: iner- and extracapillary GN.
2. According to character of inflammation: nonsuppurative exudative and proliferative.
3. According to propagation: diffuse and local.
Acute poststreptococcal glomerulonephritis
It usually appears 1 to 4 weeks after streptococcal infection of the pharynx or the skin.
In occurs most frequently in children of six to ten years of age, but adults of any age can be
affected.
Duration of disease may 1,5 to 12 monthes.
Gross appearance: Kidney enlarged; cortex broad, pale, without markings; medullary rays
congested; glomeruli just visible as grey avascular dots.
The classic diagnostic picture is one of enlarged, hypercellular, relatively bloodness
glomeruli.
The most often the histological type is intracapilary proliferative GN:
Proliferation of endothelial and mesangial cells and, in many cases, epithelial cells.
Infiltration by leukocytes, both neutrophils and monocytes. The proliferation and
leukocytes infiltration are diffuse, that is, involving all lobules of all glomeruli.
There is also swelling of endothelial cells, and the combination of proliferation,
swelling, and leukocytic infiltration obliterates the capillary lumen.
Special stains can demonstrate small deposites of fibrin within capillary lumina and
mesangium.
There may be interstitial edema and inflammation, and the tubes often contain red cell
coasts and may show evidence of degeneration.
By immunofluorescence microscopy there are glandular deposits of IgG, IgM, and C3
in the mesangium and along the basement membrane. Although present, they are often
focal and sparse. The characteristic electron microscopic findings are the discrete,
amorphous, electron-dense deposits on the epithelial side of the membrane, often
having the appearance of “humps”, presumably representing the antigen-antibody
128
complexes at the epithelial cell surface. Subendothelial and intramembranous deposits
sometimes seen, and there is often swelling of endothelial and mesangial cells.
Rapidly progressive (crescentic) glomerulonephritis
It is a syndrome characterized by the accumulation of cells in Bowman’s space in the form of
“crescents” accompanied by a rapid, progressive decline in renal function, frequently with severe
oliguria or anuria, usually resulting in irreversible renal failure in weeks or months.
Morphoplogy
According to histological picture there is extracapillary proliferative GN.
The kidneys are enlarged and pale, often with petechial hemorrhages on the cortical
surfaces.
Depending on the underlying cause, the glomeruli may show focal necrosis, diffuse or focal
endothelial proliferation.
The syndrome is characterized histologically by the accumulation of cells in Bowman’s
space in the form of “crescents”.
The histologic picture, however, is dominated by the formation of distinctive crescents,
which are formed by proliferation of parietal cells and by migration of monocytes and
macrophages into Bowman’s space. Neutrophils and lymphocytes can be present. The
crescents eventually obliterate Bowman’s space and compress the glomerular tuft. Fibrin
strands are prominent between the cellular layers in the crescents.
Electron microscopy may disclose subepithelial deposits in some cases, but in all cases
shows distinct ruptures in the GBM.
In time, most crescents undergo sclerosis.
This syndrome may occur in the course of three broad disease groups:
1. Postinfectious rapidly progressive (crescentic) glomerulonephritis, complicating
acute GN.
2. Systemic diseases (SLE, Goodpasture’s syndrom, polyarteritis nodosa,etc.).
3. Idiopathic.
Nephrotic syndrome
Membranous glomerulonephritis (MGN)
It is a major cause of nephrotic syndrome in adults.
It is characterized by the presence of electron-dense, immonoglobulin-containing deposits
along the epithelial side of the basement membrane.
In situ formation and deposition of circulating immune complexes, involving intrinsic
glomerular antigens or endogenous and exogenous or planted antigens, are postulated to
account for the subepithelial electron-dense deposits.
Early in the disease, the glomeruli may appear normal by light microscopy, but welldeveloped cases show diffuse thickening of the capillary wall.
MGN may occur in association with known disorders or etiologic agents. These include the
following:
1. Malignant epithelial tumors, particularly carcinoma of the lung and colon and
melanoma.
2. Systemic lupus erythematosus (SLE).
3. Exposure to inorganic salts (gold, mercury).
4. Drugs (penicillamine, captopril).
5. Infections (chronic hepatitis B, syphilis, schistomiasis, malaria).
6. Metabolic disorders (diabetes mellitus, thyroiditis).
In about 85% of patients, the condition is truly “idiopathic”.
Morphology
By light microscopy, the glomeruli appear normal in the early stages of the disease or
exhibit uniform, diffuse thickening of the glomerular capillary wall, hence the term
“membranous”.
By electron microscopy the apparent thickening is caused by irregular dense deposits
between the basement membrane and the overlying epithelial cells, the latter having lost
their foot processes.
129
Basement membrane material is laid down between these deposits, appearing as irregular
spikes protruding from the GMB.
In time, these spikes thicken to produce dome-like protrusions and eventually close over
the immune deposits, burying them within a markedly thickened, irregular membrane.
Immunofluorescence microscopy demonstrates that the granular deposits contain both
immunoglobulins and complement.
Others changes: protein and fatty droplets in the tubular epithelium and stroma. Foamy
macrophages and giant cells form granulomas is association with cholesterol deposits.
With progress of the disease, narrowing of the glomerular capillaries causes ischemic
atrophy of the tubules and interstitial fibrosis.
Membranoproliferative glomerulonephritis (MPGN)
As the term implies, this group of disorders is characterized histologically by alteration in
the basement membrane and proliferation of glomerular cells. Because the proliferation is
predominant in the mesangium, a frequently used synonym is mesangiocapillary GN.
Like many other GN, histologic MPGN either can be associated with other systemic
disorders and known etiologic agents (secondary MPGN) or may be primary, without
known cause (idiopathic) in the kidney.
Patients have hematuria or proteinuria demonstrate a combined nephritic-nephrotic
picture.
Morphology
Primary MPGN is devided into two major types on the basis of distinct ultrastructural,
immunofluorescent, and probably pathogenic findings.
By light microscopy both types are similar.
The glomeruli are large and hypercellular.
The hypercellularity is produced by proliferation of cells in the mesangium, although
infiltrating leukocytes and parietal epithelial crescents are present in many cases.
The glomeruli have a “lobular” appearance accentuated by the proliferating mesangial cells
and increased mesangial matrix.
The GBM is clearly thickened, often focally, most evident in the peripheral capillary loops.
The glomerular capillary wall often shows a “double-contour” or “tram-track” appearance,
especially evident in silver or PAS stains.
This is caused by “splitting” of the basement membrane because of the inclusion within it of
processes of mesangial cells extending into the peripheral capillary loops, so-called
“mesangial interposition”.
Injury of tubular structures and stroma take place.
Minimal change disease (MCD) (Lipoid nephrosis)
Nephrotic syndrom in children can be often; characterized by normal glomeruli on light
microscopy but uniform and diffuse effacement of the foot processes of visceral epithelial
cells on electronic microscopy.
Etiology is unknown.
Immunofluorescence shows no immune deposits.
The most characteristic feature of this condition is the good response to corticosteroid
therapy.
Proteinuria is usually selective and is associated with loss glomerular filtration (negative
changes) and a hyperpermeable capillary wall.
Morphology
GBM isn’t changes.
Tubules are dilated; their epithelium is swelling, conteining hyaline and fatty droplets.
Fatty degeneration, necrobiosis, atrophy, desquamation in tubular epithelium take place.
Gross appearances (“big white kidneys”): kidneys enlarged, flabby, yellow color.
Chronic glomerulonephritis (CGN)
CGN is the final stage of GN when sclerosis has eliminated many glomeruli and their
associated tubules.
This is often the late result of membranous or membranoproliferative GN, less commonly
postinfectious acute nephritis.
130
At the final stage, it is difficult to determine the etiology of the pathological lesion.
Morphology
The kidneys are symmetrically contracted and have diffusely granular, cortical surfaces.
Pieces of renal tissue adhere to stripped capsule; capsule is adherent and strips with
difficult. Weight is 50 gm each. On section, the cortex is thinned and irregular, pelvis
dilated and they’re in an increasing peripelvic fat. Such kidneys are called “secondary
shrinkage of kidneys.
The glomerular histology depends on the stage of the disease. In early cases, the glomeruli
may still show evidence of the primary disease.
Kidneys from the patients with end-stage disease on long-term dialysis exhibit a variety of
so-called “dialysis changes” that are unrelated to the primary disease. Histological feature is
nephrosclerosis.
These include arterial intimal thickening caused by accumulation of smooth muscle-like
cells and a loose, proteoglycan-rich stroma; calcification, most obvious in glomerular tufts
and tubular basement membranes; extensive deposition of calcium oxalate crystals in
tubules and interstitium; acquired cystic disease; and increased numbers or renal
adenomas and borderline adenocarcinomas.
Patients dying with chronic GN also exhibit pathologic changes outside the kidney that are
related to the uremic state and are also present in other forms of chronic renal failure. Often clinically
important, these includes uremic pericarditis, uremic gastroenteritis, secondary hyperparathyroidism
with nephrocalcinosis and renal osteodystrophy, left ventricular hypertrophy due to hypertension, and
pulmonary changes of diffuse alveolar damage often ascribed to uremia (uremic pneumonitis).
Uremia, hypertensive cardiac failure or cerebral hemorrhage may cause death.
Tubulopathy
Acute renal failure
Acute renal failure (ARF) is a syndrome associated with acute suppression of renal function,
often accompanied by oliguria, and rarely anuria or polyuria. ARF is caused by:
1. Organic vascular obstruction.
2. Severe glomerular disease.
3. Acute tubulointerstitial nephritis.
4. Massive infection.
5. Disseminated intravascular renal coagulation.
6. Urinary obstructions.
7. Acute tubular necrosis.
Acute tubular necrosis (ATN)
ATN is characterized by destrucrion of renal tubular epithelial cells either from ischemia or
nephrotoxins.
Ischemic ATN is called tubulorrhectic ATN or shock kidney, occurs due to hypoperfusion of
the kidneys resulting in focal damage to the tubules.
Etiopathogenesis
Ischemia may result from following causes:
Shock (post-traumatic, surgical, burns, dehydration, obstetrical and septic).
Crush injuries.
Non-traumatic rhabdomyolysis induced by alcohol, coma, muscle disease or extreme
muscular exertion (myoglobinuria nephrosis).
Mismatched blood transfusions, black-water fever (hemoglobinuric nephrosis).
The pathogenetic mechanism of ischemic ATN is explained on the basis of:
Arteriolar vasoconstriction induced by renin-angiotensin system.
Tubular obstruction by casts in the lumina or by interstitial edema.
Back-leak of tubular fluid into the interstitium.
Morphology
The kidneys are enlarged and swollen. On cut section, the cortex is often widened and pale,
while medulla is dark.
131
Predominant changes are seen in the tubules, while glomeruli are normal. Interstitium
shows edema and mild chronic inflammatory cell infiltrate. Tubular changes are as follows:
a) Dilatation of the proximal and distal convoluted tubules.
b) Focal tubular necrosis at different points along the nephron.
c) Flattened epithelium lining the tubules.
d) Eosinophilic hyaline casts or pigmented hemoglobin and myoglobin casts in the
tubular lumina.
e) Disruption of tubular basement membrane (tubulorrhexis).
Nephrotoxic ATN occurs as a result of direct damage to tubular cells by ingestion, injection
or inhalation of a number of toxic agents.
Etiopathogenesis
The toxic agents causing toxic ATN are:
General poisons such as mercuric chloride, carbon tetrachloride, ethylene glycol,
mushrooms and insecticides.
Heavy metals (mercury, lead, arsenic, phosphorus and gold).
Drugs, such as sulfonamides, certain antibiotics (gentamycin, cephalosporin), anaesthetic
agents (methoxyflurane, halothane), barbiturates, salicylates.
Radiographic contrast material.
The pathogenetic mechanism producing ARF in toxic ATN is in principle similar to that for
ischemic ATN.
Morphology
The kidneys are enlarged and swollen. On cut section, the cortex is pale, while the medulla
is slightly darker than normal.
In general it involves the segment of tubule diffusely. In mercuric chloride poisoning, the
features are as follows:
a) Epithelial cells of mainly proximal convoluted tubules are necrotic and desquamated
into the tubular lumina.
b) The desquamated cells may undergo dystrophic calcification.
c) Tubular basement membrane is generally intact.
d) The regenerating epithelium, which is flat and thin with few mitoses, may be seen
lining the tubular basement membrane.
The clinical course of ATN may be devided into stages:
1. The initiating stage (shock), lasting for about 36 hours, is dominated by the inciting medical,
surgical, or obstetric event in the ischemic form of ATN. Macroscopically, kidneys are diffusely
swollen and edematous. It is characterized by ischemic cortex and congestion of pyramids. Acute
renal failure and oliguria, hyperkalemia and fluid overload in patients develop.
2. The maintenance stage (Oliguric phase, 2-9 days) is characterized by sustained decreases in urine
output to between 40 to 400 ml per day, with salt and water overload, rising blood urea nitrogens,
hyperkaliemia, metabolic acidosis, and other manifestations of uremia dominating this phase. There
is blockage of renal tubules by necrotic cells, and a secondary reduction in glomerular blood flow
(caused by arteriolar constriction) reduces glomerular filtration. It stage may be fatal.
3. The recovery stage (Polyuric phase, 10-21 days) is ushered by a steady increase in urine volume
that may reach up to 3 liters per day. Regeneration of renal tubular epithelium takes place, with
removal of dead material by phagocytic cells, as well as in the form of casts in urine. As tubules open
up and glomerular blood flow increases, patients develop polyuria. This is because the regenerated
tubular cells are undifferentiated and have not developed the specializations necessary for resorption
of electrolytes and water. Replacement of fluid and electrolytes is needed to compensate for excessive
loss from urine. Hypokalemia, rather than hyperkalemia, becomes a clinical problem.
The prognosis of ATN depends on the clinical setting surrounding its development.
Tubulointerstitial Disease
The term tubulointerstitial nephritis is used for inflammatory process that predominantly
involves the renal interstitial tissue and is usually accompanied by some degree of tubular damage.
The term interstitial nephritis is reserved for those cases where there is no primary involvement of
glomeruli, tubules or blood vessels. A number of bacterial and non-bacterial, acute and chronic
conditions may produce tubulointerstitial nephritis.
132
Pyelonephritis (PN)
PN is a renal disorder affecting tubules, intrestitium, and renal pelvis and is one of the most
common diseases of the kidney. The term urinary tract infection (UTI) implies involvement of either
the bladder (cystitis) or the kidney and their collecting system (pyelonephritis), or both. UTIs are
extremely common disorders.
It occurs in two forms:
1. Acute PN is acute pyogenic infection.
2. Chronic PN is a more complex disorder: bacterial infection plays a dominant role, but other factors
(vesicoureteral reflux, obstruction) are involved in its pathogenesis.
Etiopathogenesis
The dominant etiologic agents are the gram-negative bacilli that are normal inhabitants of
the intestinal tract: E.coli (Proteus, Klebsiella and Enterobacter), Str. fecalis etc.
In most patients with UTI, the infecting organisms are derived from the patient’s own fecal
flora. This is thus a form of endogenous infection.
There are two routs by which bacteria can reach the kidneys:
a) Through the bloodstream (hematogenous).
b) From the lower urinary tract (ascending infection).
Although obstruction is an important predisposing factor in the pathogenesis of ascending
infection, it is incompetence of the vesicoureteral orifice that allows bacteria to ascend the
ureter into the pelvis.
Acute Pyelonephritis
Morphology
The hallmarks of acute PN are patchy interstitial suppurative inflammation and tubular
necrosis.
Macroscopically, the kidneys show variable numbers of small, yellowish white cortical
abscesses, which are usually spherical, under 2 mm in diameter, and are sometimes
surrounded by a zone of hyperemia; the cortical abscesses are often most prominent on the
sub-capsular surface, after the capsule has been stripped away. In the medulla the abscesses
tend to be in the form of yellowish white linear streaks that converge on the papilla. The
pelvicalyceal mucosa is hyperemic or covered with a fibrinopurulent exudate.
Histologically: the neutrophilic infiltration is limited to the interstitial tissue. Some tubules
destroyed: abscesses formed; other tubules filled by puss cells. Glomeruli usually
unaffected.
Clinical features. Classically, acute pyelonephritis has an acute onset with chills, fever, loin
pain, lumbar tenderness, dysuria and frequency of micturition. Urine will show bacteria,
pus cells and pus cell casts in the urinary sediment.
Three complications of acute PN are encountered in special circumstances.
1. Papillary necrosis is seen mainly in diabetics and in those with urinary tract
obstruction. Papillary necrosis is usually bilateral, but may be unilateral.
2. Pyonephrosis is seen when there is total or almost complete obstruction, particularly
when it is high in the urinary tract (pelvis filled with puss).
3. Perinephric abscess implies extension of suppurative inflammation through the renal
capsule into the perinephric tissue.
At the acute phase of PN, healing occurs. The neutrophilic infiltration is replaced by
macrophages, plasma cells, and (later) lymphocytes. The inflammatory foci are eventually
replaced by scars. The pyelonephritic scar is almost always associated with inflammation,
fibrosis, and deformation of the underlying calyx and pelvis.
Uncomplicated acute PN usually follows a benign course, and the symptoms disappear
within a few days after the institution of appropriate antibiotic therapy. In the presence of
unrelieved urinary obstruction, diabetes mellitus acute PN may be more serious, leading to
repeated septicemic episodes.
Chronic Pyelonephritis (CPN)
Chronic PN is a chronic tubulointerstitial renal disorder in which chronic tubulointerstitial
inflammation and renal scarring are associated with pathologic involvement of the calyces and pelvis.
133
Etiopathogenesis
Two types of chronic pyelonephritis are described:
Reflux nephropathy. Reflux of urine from the bladder into one or both the ureters during
micturition is the major cause of chronic pyelonephritis. Vesicoureteric reflux is particularly
common in children, especially in girls, due to congenital absence or shortening of the
intravesical portion of the ureter so that ureter is not compressed during the act of
micturition. Reflux results in increase in pressure in the renal pelvis so that the urine is
forced into renal tubules, which are eventually followed by damage to the kidney and scar
formation.
Obstructive pyelonephritis. Obstruction to the outflow of urine at different levels
predisposes the kidney to infection. Recurrent episodes of such obstruction and infection
result in renal damage and scarring.
Morphology
Gross examination. The kidneys are usually small and contracted (weighing less than 100
gm) showing unequal reduction; if bilateral, the involvement is asymmetric. The surface of
the kidney is irregularly scarred; the capsule can be stripped off with difficulty due to
adherence to scars. There is generally dilatation of pelvis and blunted calyces. This
contrasts with chronic glomerulonephritis, in which the kidneys are diffusely and
symmetrically scarred.
The microscopic changes involve predominantly tubules and interstitium.
The tubules show atrophy in some areas and hypertrophy in others, or dilatation. Dilated
tubules may be filled with colloid crystals, producing thyroidisation of tubules (thyroidlike).
Interstitium. There is chronic interstitial inflammatory reaction, chiefly composed of
lymphocytes, plasma cells and macrophages with pronounced interstitial fibrosis.
Xanthogranulomatous pyelonephritis is an uncommon variant characterised by collection
of foamy macrophages admixed with other inflammatory cells and giant cells.
Pelvicalyceal system. The renal pelvis and calyces are dilated. And show marked
chronic inflammation and fibrosis.
Blood vessels. Blood vessels entrapped in the scarred areas show obliterative endarteritis.
Glomeruli. There is often periglomerular fibrosis. In advanced cases, there may be
hyalinisation of glomeruli.
Clinical features. Chronic pyelonephritis often has an insidious onset. The patients present
with clinical picture of chronic renal failure or with symptoms of hypertension.
Chronic obstructive PN may be insidious in onset or may present the clinical
manifestations of acute recurrent PN with back pain, fever, frequent pyuria, and
bacteriuria.
Infections of the lower urinary tract
Infections in the lower urinary tract are predisposed by obstruction and stasis.
Lower urinary tract infection is usually due to Gram-negative coliform bacilli, e.g. E. coli
and Proteus, which are normally in the large bowel; because they have a short urethra,
women are particularly prone to developing ascending infections.
In men, lower urinary tract infection is usually associated with structural abnormalities of
the lower urinary tract and stasis due to obstruction.
Diabetes mellitus also predisposes to infection.
Morphology
The pelvicalyceal system is dark reddish brown as a result of acute inflammation of the
usually smooth creamy mucosal lining due to bacterial infection.
The kidney is also congested and some small scattered abscesses are present in the cortex
and medulla (acute pyelonephritis).
Obstruction of the drainage of urine from the kidney causes hydronephrosis.
Obstruction, one of the most important consequences of disease of the lower urinary tract,
may occur at any place in the tract: renal pelvis (calculi, tumors), pelviureteric junction
(stricture, calculi, extrinsic compression), ureter (calculi, extrinsic compression -pregnancy,
tumor, fibrosis), bladder (tumor, calculi); urethra (prostatic hyperplasia or carcinoma,
urethral valves, urethral stricture).
134
If obstruction occurs in the urethra, the bladder develops dilatation and secondary
hypertrophy of muscle in its wall. This predisposes to development of out pouching of the
bladder mucosa (diverticulae).
If obstruction occurs in a ureter, there is dilatation of the ureter (megaureter), with
progressive dilatation of the renal pelvicalyceal system, termed hydronephrosis. Fluid
entering the collecting ducts cannot empty into the renal pelvis and there is intrarenal
resorption of fluid. At this stage, if the obstruction is relieved, renal, function returns to
normal. However, if obstruction persists, there is atrophy of renal tubules, glomerular
hyalinization, and fibrosis. As an end-stage, the renal parenchyma becomes severely
atrophic and renal function is permanently impaired.
Urinary tract obstruction also predisposes to infection and stone formation.
Urolithiasis
Urolithiasis or formation of urinary calculi at any level of the urinary tract is a common
condition. It is estimated that approximately 2% of the population experiences renal stone
disease at sometime in their life with male-female ratio of 2:1.
Renal calculi are characterized clinically by colicky pain (renal colic) as they pass down
along the ureter and manifest by hematuria.
Sites of formation. Two suggestions have been made:
1. Precipitates form in the collecting tubules and pass into renal pelvis where they
enlarge.
2. Deposits are formed in the lymphatic vessels of the renal papillae and are extruted
into the renal pelvis.
Types of Urinary Calculi
There are 4 main types of urinary calculi:
1) Calcium stones. Calcium stones are the most common comprising about 75% of all
urinary calculi. They may be pure stones of calcium oxalate (50%) or calcium phosphate
(5%), or mixture of calcium oxalate.
2) Mixed (Struvite) stones. About 15% of urinary calculi are made of magnesiumammonium-calcium phosphate, often called struvite. “'Staghorn stone” which is a large,
solitary stone that takes the shape of the renal pelvis where it is often formed is an
example of struvite stone.
3) Uric acid stones. Uric acid calculi are radiolucent unlike radio-opaque calcium stones.
Uric acid stones are smooth, yellowish-brown, hard and often multiple.
4) Cystine stones. Cystine stones are small, rounded, smooth and often multiple. They are
yellowish and waxy. They are seen in heritable tubular transport defects causing
cystinuria.
Complications: pyelonephtitis, hemorrhage, hydronephrosis.
Hydronephrosis
Hydronephrosis is the term used for dilatation of renal pelvis and calyces due to partial or
intermittent obstruction to the outflow of urine.
Hydroureter nearly always accompanies hydronephrosis
Hydronephrosis may be unilateral or bilateral.
Unilateral hydronephrosis. This occurs due to some form of ureteral obstruction at the
level of periureteric junction (PUJ). The causes are:
a) Intraluminal, e.g. a calculus in the ureter or renal pelvis.
b) Intramural, e.g. congenital PUJ obstruction, atresia of ureter, inflammatory stricture,
trauma, neoplasm of ureter or bladder.
c) Extramural, e.g. obstruction of upper part of ureter by inferior renal artery or vein,
pressure on ureter from outside such as carcinoma cervix, prostrate, rectum, colon or
cecum and retroperitoneal fibrosis.
Bilateral hydronephrosis. This is generally the result of some form of urethral obstruction
but can occur from the various causes listed above if the lesions involve both sides.
a) Congenital, e.g. atresia of the urethral meatus, congenital posterior urethral valve.
135
b) Acquired, e.g. bladder tumor involving ureteric orifices, prostatic enlargement,
prostatic carcinoma and prostatitis, bladder neck stenosis, inflammatory or traumatic
urethral stricture and phimosis.
Morphology
The kidneys may have moderate to marked enlargement.
Initially, there is extrarenal hydronephrosis characterised by dilatation of renal pelvis
medially in the form of a sac.
Eventually, the dilated pelvi-calyceal system extends deep into the renal cortex so that a
thin rim of renal cortex is stretched over the dilated calcyes and the external surface
assumes tabulated appearance. This advanced stage is called as intrarenal hydronephrosis.
The wall of hydronephrotic sac is thickened due to fibrous scarring and chronic
inflammatory cell infiltrate.
Cystic disease of the kidney
There are several cystic diseases of the kidney, some of which produce renal failure by
causing disturbance of renal structure. Importantly, some conditions are heritable.
Adult polycystic disease is inherited in an autosomal dominant trait, generally becoming
clinically manifest in adult life. Increasingly, disease is detected in childhood, with family
screening and ultrasound examination.
Cysts develop and progressively enlarge over a number of' years, but remain asymptomatic
until the number and size of the cysts is so great that the patient becomes aware of
abdominal masses.
At about the same time, the replacement and compression of functioning renal parenchyma
by the cysts leads to slowly progressive impairment of renal function, and patients develop
chronic renal failure and hypertension.
Patients with adult-type polycystic renal disease may also develop cysts in the liver, lung
and pancreas. There is an association with berry aneurysms of the cerebral arteries, which,
with development of hypertension, predisposes to intracranial hemorrhage.
Infantile polycystic disease is uncommon and is encountered at birth. Children develop
severe renal failure, with compression of the lungs due to massive enlargement of the
kidneys.
Simple renal cysts are the most common form of renal cystic disease and must be
distinguished from the congenital types discussed above. They are widely held to be
acquired abnormalities, incidence increasing with age. They contain clear, watery fluid and
have a smooth lining.
Simple cysts may be single or multiple and vary in size, generally being no larger than 5-6
cm. They have no effect on renal function, but may rarely become infected or develop
hemorrhage.
Acquired cystic disease is seen in kidneys left in situ while patients are treated by dialysis or
transplantation for chronic renal failure. The kidney is converted into a mass of large cysts.
Hemorrhage into cysts is common, leading to bloodstained contents.
Chronic renal failure
Nephrosclerosis is morphologic basis of chronic renal failure.
Uremia is a syndrome encompassing a group of clinical and biochemical sings derived
essentially from the retention of waste products and the failure to control fluid and
electrolyte balance.
Uremia is final stage of chronic renal failure, which is characterised by
1. Hypernitrogenemia.
2. Metabolic acidosis (accumulation of sulphates, phosphates, and organic acids).
3. Hyperkaliemia, hypercalcemia.
4. Anemia.
5. Depression of immunological reaction. Infections are common and will in turn affect
renal function.
6. Arterial hypertension.
7. Hemorrhagic syndrome (petechias, hemorrhagic erosions and ulcer in mucosa).
8. Fibrinous inflammation:
136
a) Fibrinous pericarditis (“cor vilosum”).
b) “Uremic pneumonitis” with pleural exudates.
c) Uremic gastritis, enteritis, colitis.
d) Edema of lungs.
The prognosis of final stage renal failure has been greatly improved by dialysis, renal
transplantation.
GENITAL TPACT DISEASES
Diseases of Cervix
The cervix is an important site of pathology, particularly in women of reproductive age.
The ectocervix is covered by squamous epithelium, and the endocervical canal is covered by
mucus-secreting columnar epithelium, which shows glandular down growth.
At various stages in a woman’s reproductive life, the junction between the squamous and
columnar epithelium migrates into the convexity of the ectocervix, then back into the
endocervical canal. This squamocolumnar junction is the seat of most of the epithelial
diseases that occur in the cervix.
Cervical erosion (endocervicosis)
It represents an unfolding and eversion of the distal endocervix into an ectocervix. The term
“cervical ectopia” is preffered.
Etiology: increased uterine bulk, in pregnancy, hormonal stimulation.
According to duration the endocervicosis classified:
1. Simple endocervicosis is characterized by metaplasia of the squamous epithelium into
columnar epithelium without proliferation of reserve cells, presence of the cervical
glands in ectocervix and papillary formation.
2. Progressive endocervicosis is characterised by proliferation of reserve cells and
presence of the various size glands. Zone transformation is dilated.
3. Healing endocervicosis is characterized by recovery of normal structure of ectocervix
or formation of Naboti’s cysts. Due to impairment of differentiations the dysplasia can
take place.
Dysplasia
Dysplasia refers as cervical intraepithelial neoplasia (CIN) and it has 3 grades of
differentiation:
1. CIN 1 or mild dysplasia: cells of basal third have high nucleocytoplasmic ratio and
pleomorphic nuclei.
2. CIN 2 or moderate dysplasia: basal cells occupy lower half of squamous epithelium.
3. CIN 3 or severe dysplasia or cancer in situ: almost complete loss of stratification,
loss of polarity of the cells, variation in nuclear size with increase in
nuclear/cytoplasmic ratio and mitotic figures.
Cervicitis
Cervicitis may be specific and non-specific.
Acute and chronic cervicitis results from infection by any number of microorganisms,
particulary Streptococcus, staphylococcus, or Enterococcus, and, less commonly, Neisseria
gonorrhoeae and Chlamydia trachomatis.
Some of these microorganisms are sexually transmitted, whereas others may be introduced
by foreign bodies, such as residual fragments of tampons and pessaries.
Purulent inflammation is a clinical sign of acute cervicitis. The inflamed cervix becomes
congested and edematous.
Since biopsy samples from the cervix frequently exhibit some degree of nonspecific chronic
inflammation, the diagnosis of chronic cervicitis should be made only when numerous
lymphoid cells are present. Leukoplakia may develop (it means the white patches of
hyperkeratosis).
Diseases of Endometrium
137
Dysfunctional Bleeding
Dysfunctional uterine bleeding is denned as abnormal bleeding in the absence of an organic
lesion of the endometrium.
It is one of the most common gynecologic disorders of women of reproductive age, but one
that is still poorly understood.
The bleeding may be due to anovulatory cycles related to excessive and prolonged
estrogenic stimulation.
Without ovulation, a corpus luteum does not develop and progesterone is not secreted.
The endometrium, therefore, fails to proceed through the normal secretory phase, and an
abnormal menstrual cycle results.
Organic lesions of the uterus must be excluded before the diagnosis of dysfunctional
bleeding can be made. Examples of organic disorders are carcinoma, hyperplasia, polyps,
endometritis, and complications of intrauterine or ectopic pregnancy.
Anovulatory Bleeding
Anovulatory bleeding is the most common form of dysfunctional uterine bleeding,
particularly during adolescence and the climacteric period.
It is believed that estrogen maintains the stromal fluid turgescence that supports the blood
vessels.
Anovulatory bleeding is caused by a fall in estrogen levels, which results in loss of fluid from
the stroma and hence loss of vascular support. The vascular collapse leads to compression
of the vessels, which in turn leads to stasis, thrombosis, infarction, and hemorrhage.
On microscopic examination the glands are disordered and appear crowded because of
severe stromal necrosis and collapse of the proliferative endometrium.
Abnormalities of the Normal Menstrual Cycle
Dysfunctional bleeding may also be associated with abnormalities of the normal menstrual
cycle.
Ovulatory oligomenorrhea (cycle longer than 45 days) is almost always due to a long
follicular phase and may be the prelude to ovarian failure.
Ovulatory polymenorrhea, in which cycles are less than 18 days in length, is caused by short
follicular phases (seen generally in adolescence) or short luteal phases (inadequate luteal
phase). The latter may be due to defects in factors that maintain the corpus lutein.
Endometritis
Acute endometritis
This is almost confined to infection associated with partirition and abortion.
A mixed bacterial flora, pyococci, coliform organisms and proteus are usual.
Suppurative inflammation is usual.
Presence of polymorphonuclear leukocytes, results when an infection ascends from the
cervix.
Curettage is both diagnostic and curative because it removes the necrotic tissue that serves
as the nidus of infection.
Complications may follow endometritis: myometritis, parametritis, salpingitis, peritonitis,
subsequent tubal blockage and infertility.
Pyometra, pus in the endometrial cavity, is associated with any lesion that causes cervical
stenosis, such as a tumor or scarring from surgical treatment (conization) of the cervix.
Long-standing pyometra may be associated with the development of squamous cell cancer
of the endometrium.
Chronic endometritis
Chronic endometritis is usually associated with recent gestation, pelvic inflammatory
disease, intrauterine contraceptive devices (IUD) use, and retained products of conception
after an abortion or delivery, menstrual irregularities, but is also found in women who are
being investigated for infertility.
Chronic endometritis may be caused by gonococcal or chlamydial infection, or tuberculosis.
Clinically, patients usually complain of bleeding, pelvic pain, or both.
138
Histologically: stroma infiltrated by plasma cells and lymphocytes; glands small and
infrequent; epithelium atrophied.
Hyperplasia of endometrium
Endometrial hyperplasia usually results with conditions of prolonged estrogen excess and can
lead to metrorrhagia (uterine bleeding at irregular intervals), menorrhagia (excessive bleeding with
menstrual periods), or menometrorrhagia.
It is classified into following 3 types:
1. Simple hyperplasia (cystic glandular hyperplasia) is characterised by the presence of large
and cystically dilated, varying-sized glands, which are lined by atrophic epithelium. Simple
endometrial hyperplasias can cause bleeding, but are not thought to be premalignant.
2. Adenomatous hyperplasia (complex hyperplasia without atypia). This shows distinct
proliferative pattern. The glands are increased in number, exhibit variation in size and are
irregular in shape. Multiple layers of tall columnar epithelial cells with large nuclei, which
have not lost basal polarity, line the glands and there is no atypia. Adenomatous hyperplasia
is premalignant.
3. Atypical hyperplasia (complex hyperplasia with atypia) is characterised by the presence of
atypical cells in the hyperplastic epithelium. The glands are enlarged and irregular with
columnar cells that have some atypia (loss polarity, large size, irregular and hyperchromic
nuclei, prominent nuclei, and altered nucleocytoplasmic ratio).
Endometriosis
When endometrial glands and stroma are found outside the uterus, the condition is known
as endometriosis.
Up to 10% of women may have this condition. It can be very disabling and painful, even
when just a few foci are present.
Typical locations for endometriosis may include: ovaries, uterine ligaments, rectovaginal
septum, pelvic peritoneum, fallopian tubes and laparotomy scars. Endometriosis may even
be found at more distant locations such as appendix and vagina.
Grossly, in areas of endometriosis the blood is darker and gives the small foci of
endometriosis the gross appearance of "powder burns". Such areas of endometriosis can
be seen and obliterated by cauterization via laparoscopy. Sometimes the old dark brown
blood collects over time from repeated hemorrhage in a cystic space in the ovary and
produces a so-called “chocolate cyst” (endometriotic cyst).
Histologically: foci of endometrial glands and stroma, old or new hemorrhage,
hemosiderin-laden macrophages and surrounding zone of inflammation and fibrosis.
Diseases of Fallopian tubes
Acute and chronic salpingitis usually results from an ascending infection from the lower
genital tract.
The most common causative organisms are E. coli, N. gonorrheae, Chlamydia, and
Mycoplasma. Clostridia perfringens and various other anaerobes are less commonly
encountered.
A fallopian tube damaged by prior infection is particularly susceptible to reinfection.
Acute Salpingitis
The host responds with a brisk granulocytic infiltrate and vascular engorgement, and edema of
the involved tubal layers ensues. As the lumen fills with granulocytes, the tube distends and
pyosalpinx develops.
Chronic Salpingitis
Chronic salpingitis usually results from repeated episodes of acute salpingitis.
The acute episodes, particularly those associated with chlamydial infection, may be
asymptomatic.
Complications may be caused by either destruction of epithelium or deposition of fibrin on
the mucosal plicae; the fibrin bridges cause the plicae to adhere to one another.
139
Fibrinous adhesions between the serosa and surrounding peritoneal surfaces may organize
into thin, fibrous adhesions (“violin string” adhesions).
In severe chronic salpingitis, the adhesions may be dense and the fimbria adhere to each
other to form a blunted; clubbed end.
Ovarian involvement leads to the formation of a tubo-ovarian abscess.
The consequence of the blocked lumen may be a hydrosalpinx or pyosalpinx. Because of
destruction of the tubal epithelium and fibrosis, chronic salpingitis may lead to infertility
and ectopic pregnancy.
Diseases of Ovaries
Ovarian changes of functional origin.
1. Follicilar cysts are cysts arising from Graafian follicles and are lined by granulosa cells,
with an outer coat of thecal cells. They filled with clear serous fluid and may attain a diameter up to 2
sm. They may be single or multiple. Multiple follicular cysts, usually small, are associated with
endometrial hyperplasia.
2. Luteal cysts are cysts from which the granulosa cells have disappeared, leaving cysts
surrounded by luteinised tissue. Cysts are typically 2-3 cm in diameter, with a thick, yellow lining of
luteinized granulosa cells. There is continued production of progesterone, resulting in menstrual
irregularity.
3. Theca lutein cysts are usually seen as multiple bilateral cysts, up to 1 cm in diameter, filled
with clear fluid. They are caused by high levels of gonadotropin, which precipitates follicle
development (e.g. in hydatidiform mole and drug treatment).
4. Polycystic ovary disease (Stein-Leventhal Syndrome) is associated with obesity,
hirsutism, oligomenorrhea, anovulation, and infertility.
The pathogenesis of this syndrome is still uncertain. Patients have a persistent anovulatory
state, high level of estrogen, low level of progesteron with high levels of circulating
androgen produced by the ovary. The high estrogen levels may cause endometrial
hyperplasia and increase the risk of development of endometrial carcinoma.
The ovaries are usually involved bilaterally and are at least twice the size of the normal
ovary. They are grey-white color and studded with multiple small bluish cysts just beneath
the cortex.
Histologically. The outer cortex is thick and fibrous. The subcortical cysts are lined by
prominent luteinised theca cells and represent follicles in various stages of maturation but
there is no evidence of corpus lutein.
140
Obstetric pathology
Pre-eclampsia and eclampsia
Pre-eclamptic toxemia syndrome is characterised by hypertension, proteinuria and
peripheral edema.
Seen particularly in association with multiple pregnancies, primigravidae and women
over the age of 35 years.
Most cases are mild, with the blood pressure under 100 mmHg diastolic and no
proteinuria; in severe cases the diastolic pressure is consistently above 100 mmHg, and
there is proteinuria and severe peripheral edema.
A feature of pre-eclampsia is reduced placental blood flow; this may lead to fetal hypoxia
in late pregnancy, particularly during labour, with increased risk of perinatal mortality.
The fetus may also suffer intrauterine growth retardation and have low birth weight.
Placental ischemia takes place.
In the kidney, endothelial cells become swollen, with deposition of fibrin in glomeruli,
leading, to proteinuria. If untreated, severe hypertension and intravascular coagulation
occur with development of cerebral ischemia and fits.
Eclampsia is now a fare complication of pregnancy. Patients develop severe systemic
disturbance, rapid and sustained rise in blood pressure, shock, anuria and fits.
Complications and causes of death: patients develop disseminated intravascular
coagulation, with widespread occlusion of blood vessels, fibrinoid necrosis of vessel wails,
and, in fatal cases, widespread microinfarcts in brain, liver, kidney and other organs.
Ectopic Pregnancy
Ectopic pregnancy refers to any gestation that develops outside the endometriun.
Over 95% occur in the tube (ampullary implantation, interstitial implantation and tubal
wall).
Ovarian pregnancy is presumed to result from the rare fertilization and trapping of the
ovum within the follicle just at the time of its rupture.
Abdominal pregnancies may develop when the fertilized ovum drops out of the fimbriated
end of the tube.
Etiology of tubal pregnancy: salpingitis, leading to partial blockage of the tube; in women
fitted with intrauterine contraceptive devices, endometriosis. Other factors are peritubal
adhesions owing to appendicitis, leiomyomas, and previous surgery.
In all abnormal locations, the fertilized ovum undergoes its usual development with the
formation of placental tissue, amniotic sac, and fetus, and the host implantation site
develops decidual changes and syncytio-trophoblasts.
Abdominal pain is the most common symptom.
The appearance of ectopic pregnancy resembles that of placenta increta and percreta of
the uterus. Because the tubal mucosa has a limited ability to undergo decidualization, the
trophoblast readily penetrates the mucosa and wall, a situation, which results in an
abnormal implantation.
The wall of the fallopian tube is thin and unless the ectopic pregnancy is discovered, the
wall usually ruptures by the 12th week of gestation.
Tubal rupture with subsequent hemorrhage is a life-threatening complication.
The direction of rupture varies:
1. Rupture into the lumen of the tube and leakage into perinatal cavity (tubal abortion).
Exceedingly rarely the whole pregnancy – ovum and placental tissue – aborts into
peritoneal cavity where it reimplants. Usually development is limited and the fetus
dies.
2. Rupture directly into the peritoneal cavity. If the implatation is interstitial, there may
be a further complication – damage to the uterine arteries with arterial bleeding.
3. Rupture into the broad ligament lead to extraperitoneal hematoma.
Gestational Trophoblastic Disease
The clinical term “gestational trophoblastic disease” include of hydatidiform mole (complete
and partial mole), invasive mole, gestational choriocarcinoma, and placental site trophoblastic tumor.
141
Hydatidiform Mole
1. Complete type.
In complete hydatidiform mole, grossly swollen chorionic villi, which resemble a bunch of
grapes, show varying degrees of trophoblastic proliferation.
There is no embryo.
Complete mole results from fertilization of an egg in which the maternal chromosomal
material has been lost or inactivated by a single sperm with a 23X set of chromosomes,
which duplicate to 46XX.
The embryo dies at an early stage before the placental circulation has developed, and
chorionic villi then contain few, if any, blood vessels.
Complications of complete hydatidiform mole include uterine hemorrhage, coagulopathy,
uterine perforation, trophoblastic embolism, and infection. The most important
complication is the development of choriocarcinoma.
2. Partial type.
In partial hydatidiform mole two populations of chorionic villi exist, some of which show
hydropic swelling.
Trophoblastic proliferation is focal and usually less pronounced than in the complete
mole.
In partial hydatidiform mole, unlike complete mole, there is frequently an associated
embryo.
Partial hydatidiform mole is generally the result of fertilization of an egg by two paternal
sets of chromosomes, with the maternal chromosomes remaining. This results in triploidy.
The fetus associated with a partial mole usually dies at approximately 10 weeks of
gestation, and the mole is aborted shortly thereafter.
Microscopically, partial hydatidiform mole resembles complete mole, being composed of
seemingly normal small villi along with villi that have accumulated considerable fluid.
Blood vessels are typically found within the chorionic villi and contain fetal (nucleated)
red blood cells. The villous outlines commonly have a scalloped appearance.
It is difficult to determine the relative frequency of complete and partial moles, since the
entity of partial mole has only recently been recognized. Partial moles have a lower
malignant potential than complete moles.
3. Invasive type.
The invasive mole, also called chorioadenoma destruens, is a hydatidiform mole that has
invaded the underlying myometrium.
Uterine perforation from the locally infiltrative disease is the major complication.
Theca lutein cysts (hyperreactio luteinalis) may occur with any form of trophoblastic
disease and may be prominent with invasive moles.
Microscopically, invasive moles show less hydropic change than complete moles;
trophoblastic proliferation is usually prominent.
Benign diseases of the breast
Fibrocystic disease
Fibrocystic disease is the most common disorder of the female breast.
The cause of fibrocystic disease is uncertain. Most believe that it is due to disturbances of
cyclical ovarian estrogen and progesterone levels, accompanied by altered responsiveness
of breast tissues in women approaching the menopause.
Fibrocystic change is characterized by hyperplastic overgrowth of components of the
mammary unit, i.e. lobules, ductules and stroma.
In this condition there are four characteristic features, and the form, which the disease
takes varies according to the relative proportions of these features:
1. Fibrosis. This is mainly an increase in the amount of collagen rather a true growth of
fibrous tissue.
2. Adenosis.
This is an increase in the number of lobules and in the size of existing lobules.
Sclerosing adenosis (fibroadenomatoid hyperplasia or fibroadenosis) is localised
condition, which may simulate carcinoma.
There is proliferation of acini and stroma, and mitotic activity can be marked but there is
no danger of malignancy.
142
This presents as palpable thickening and nodularity of breast tissue, but may also result in
the development of single breast lumps.
3. Cyst formation.
Cysts are a prominent component, increasing in incidence with the approach of the
menopause.
Obstruction of ducts leads to dilatation on the ducts and acini.
They range in size from those detectable only by histology to palpable lesions I-2 cm in
diameter.
Histologically: cysts are lined by flattened epithelium derived from the lobular-ductal unit
and are filled with watery fluid. As some carcinomas of the breast may be associated with
cysts, it is not safe to assume that a lesion is benign because it has a fluid-filled cyst. The
lining epithelium may show apocrine metaplasia.
4. Fibrocystic change.
Epithelial hyperplasia is the most important component because it forms a link between
simple proliferation and malignant change.
Macroscopically, areas of fibrocystic changes appear as firm, rubbery replacement of breast
tissue, in which cysts may be visible.
There are many histological variations within fibrocystic disease, such as:
a) Proliferation of myoepithelial layer;
b) Proliferation of ductal epithelium forming an irregular network (atypical ductal
hyperplasia);
c) Uniform proliferation of acinar epithelium without acinar expansion (atypical
lobular hyperplasia).
Fibroadenoma
Fibroadenoma is a bening nodular proliferation, now considered to be a component of
fibrocystic changes and not a true neoplasm. The fibroadenoma is therefore best regarded
as a form of hormone-dependent nodular hyperplasia, rather than a true benign tumor.
Fibroadenoma presents as a mobile lump in the breast of young women.
Macroscopically, fibroadenomas are typically 1-3 cm in diameter, appearing as firm,
rubbery, well-circumscribed, elastic consistency, glistening, greish cut surface.
Histologically:
a) Small acinar and duct structures resembling normal brest.
b) Fibrous tissue arranged around acini.
c) Epithelium forms clefts: these are due to pressure from the projecting fibrous
tissue.
Diseases of male genitalia
Prostatitis may be acute, chronic and granulomatous types.
Acute prostatitis
Acute prostatitis is characterised by acute focal or diffuse suppurative inflammation of the
prostate.
It occurs most commonly due to ascent of bacteria from the urethra, less often by descent
from the upper urinary tract or bladder, and occasionally by lymphogenous or
hematogenous spread from a distant focus of infection.
The infection may occur spontaneously or may be a complication of urethral
manipulation such as by catheterization, cystoscopy, etc.
Macroscopically, the prostate is enlarged, swollen and dense. Cut section shows multiple
abscesses and foci of necrosis.
Histologically, the prostatic acini are dilated and filled with neutrophilic exudate. There
may be diffuse acute inflammatory infiltrate. Edema, hyperemia and foci of necrosis
frequently accompany acute inflammatory involvement.
Chronic prostatitis
Macroscopically, the prostate may be enlarged, fibrosed and shrunken.
Microscopically, the diagnosis of chronic prostatitis is made by foci of lymphocytes,
plasma cells, macrophages and neutrophils within the prostatic substance. Prostatic
143
calculi and foci of squamous metaplasia in the prostatic acini may accompany
inflammatory changes.
Granulomatous prostatitis is a variety of chronic prostatitis, probably caused by
leakage of prostatic secretions into the tissue, or could be of autoimmune origin.
Macroscopically, the gland is firm to hard, giving the clinical impression of psoriatic
carcinoma on rectal examination. Microscopically, the inflammatory reaction consists of
macrophages, lymphocytes, plasma cells and some multinucleated giant cells.
Bening prostatatic hyperplasia (Nodular hyperplasia)
Nodular prostatic hyperplasia has been suggested by some as precursor for development of
prostatic cancer.
Morphology
Macroscopically:
The enlarged prostate is nodular, smooth and firm.
The appearance on cut section varies depending upon whether the hyperplasia is
predominantly of the glandular or fibromuscular tissue.
In primarily glandular benign nodular hyperplasia the tissue is yellow-pink, soft, honeycombed, and milky fluid exudes.
In mainly fibromuscular benign nodular hyperplasia the cut surface is firm, homogeneous
and does not exude milky fluid.
The hyperplastic nodule forms a mass mainly in the inner periurethral prostatic gland so
that the surrounding prostatic tissue forms a false capsule, which enables the surgeon to
enucleate the nodular masses.
Microscopically:
Hyperplasia of all tissue elements in varying proportions - glandular, fibrous and
muscular take place.
Glandular hyperplasia predominates in most cases and is identified by exaggerated intraacinar papillary infoldings with delicate fibrovascular cores.
Fibromuscular hyperplasia when present as dominant component appears as aggregates
of spindle cells forming an appearance akin to fibromyoma of the uterus.
Complications
Chronic retention of urine.
Cystitis and pyelonephritis.
Hydronephrosis.
Bladder stone.
Gynecomastia of male breast
Gynecomastia of male breast is most commonly idiopathic, but may be a sign of
underlying endocrine disturbance.
The male breast is normally rudimentary and inactive, consisting of fibroadipose tissue
containing atrophic mammary ducts.
Enlargement of the male breast, which is termed gynecomastia, may be unilateral (70% of
cases) or bilateral.
In most cases it is idiopathic. Other causes include: Klinefelter’s syndrome. Estrogen
excess (cirrhosis, puberty, adrenal tumor, exogenous estrogens), gonadotropin excess
(testicular tumor), prolactin excess (hypothalamic or pituitary disease), drug-related
(spironolactone, chlorpromazine, digitalis).
Macroscopically, there is enlargement of the breast as a firm, raised, rubbery mass
beneath the nipple.
144
ENDOCRINE PATHOLOGY
The endocrine system consists of a highly integrated and widely distributed group of
organs whose primary function is the control of homeostases.
All peripheral endocrine glands (thyroid, adrenal, pancreas, sexual, parathyroid) are
closely connected with each other as well as with the central endocrine glands (pituitary,
epiphysis) and neuronal (hypothalamus).
All diseases of the endocrine system are divided into 1) congenital, 2) acquired.
They may be represented by
1. Hypofuniction.
2. Hyperfunction.
3. Dysfunction.
In this case, dystrophy, atrophy, dysplasia, sclerosis and tumors may develop.
A practical classification of endocrine pathology is based on the damage of the main
(primary) gland. The most frequent are endocrinopathy of
1. Pituitary body.
2. Adrenal glands.
3. Thyroid gland.
4. Pancreas.
5. Parathyroid gland.
6. Sexual glands.
Diseases of pituitary body
Diseases of pituitary body may occur the symptoms of hyperpituitarism or hypopituitarism.
Hyperpituitarism is characterised by oversecretion of one or more of the pituitary
hormones due to the development of a hormone- secreting pituitary adenoma. The most frequent
diseases of pituitary body associated with hyperfunction are Itsenko-Cushing disease, acromegaly,
and gigantism.
1. Itsenko-Cushing disease.
Itsenko-Cushing disease occurs in adenomas from basophilic cells of anterior lobe of the
pituitary or adenocarcinoma in rare cases. Increased ACTH production causes cortex
hyperplasia as well as increased production of glucocorticoids.
It results in obesity of face and body, elevation of arterial pressure, diabetes mellitus, and
sexual gland dysfunction. Osteoporosis, nephrolithiasis and chronic pyelonephritis may
also develop.
The disease should be differentiated from Itsenko-Cushing syndrome. Its clinical
manifestations (so called Cushingoid) are the same as in the disease (obesity of the upper
part of the body), but the other signs are not clearly marked.
The causes of these states are adrenals damage (tumor of zona fasciculata), the
administration of hormones (cortisole, prednisolone, hydrocortisone).
2. Acromegaly and gigantism
Excess of STH stimulates all mesenchymal derivatives (bones, cartilages, connective
tissue).
If the disease occurs in adults, it is called acromegaly (the bones does not grow but ears,
nose, lower jaw, feet and hands enlarge). The term “acromegaly” means increased growth
of extremities.
If the disease occurs in prepubertal boys and gerls, it is called gigantism.
The other glands also involve by the process (goiter, atrophy of insulin apparatus of
pancreas, thymus and epiphysis hyperplasia, adrenal cortex hyperplasia, sexual glands
atrophy occur).
Hypopituitarism is characterised by less secretion of one or more of the pituitary hormones
due to destraction of the anterior lobe (by metastases, ischemic necrosis) and the development of a
nonsecretory adenoma or others tumors. The most frequent diseases of pituitary body associated with
hypofunction are hypophyseal nanism, cerebro-hypophyseal cachexia, and diabetes insipidus.
1. Pituitary dwarfism (nanism) develops in congenital hypoplasia of the pituitary
body or its necrosis in children. General underdevelopment of the organism with
preserved proportions is observed.
2. Simmonds’ disease (cerebro-hypophyseal cachexia) is caused by necrosis of
pituitary anterior lobe. It may occur after childbirth due to vascular embolism as well
as due to syphilis, tuberculosis, and tumor. It manifests by cachexia, inner organ
145
atrophy, and sexual dysfunction.
3. Diabetes insipidus is caused by tumors, inflammation, sclerosis, and trauma of the
posterior lobe of pituitary. It manifests itself by increased urine excretion due to
deficiency of Antidiuretic hormone.
Diseases of adrenal glands
Adrenal glands consist of cortex and medullar substance. There are 3 zones in the cortex:
glomerular zone which produces mineralocorticoids e.g. aldosterone, zona fasciculata
which produces glucocorticoids, reticular zone which produces sexual hormones.
The most frequent disease associated with hypoadrenalism is Addison’s disease.
In 1849 Addison described the so-called bronze disease, which develops in bilateral lesion
of adrenal cortex with the development of acorticism (absence of hormones) or
hypoadrenocorticosis.
The causes of Addison’s disease are divided into two groups:
1. One of them causes primary Addison’s disease (genetic autoimmune disturbances).
2. Secondary Addison’s disease is caused by metastases in the adrenal glands,
amyloidosis, hemorrhage, tuberculosis; necrosis due to vascular thrombosis, damage of
the pituitary body (decreases ACTH or corticotropin releasing factor).
Morphology
Hyperpigmentation of the skin and mucous membrane due to excessive production of
melanin stimulating hormone.
Myocardial atrophy.
Changes of the lumen in the aorta and large vessels.
Hyperplasia of the cells of islets of Langerhans in the pancreas (hypoglycemia).
Gastric mucosa atrophy.
Hyperplasia of thymus and lymphatic peripheral tissue.
The cause of death:
Acute adrenal failure.
Cachexia (suprarenal cachexia)
Cardiovascular insufficiency.
Diseases of theThyroid gland
Two significant functional disorders characterised by distinct clinical syndromes are discribed.
There are: hyperthyroidism (thyrotoxicosis) and hypothyroidism (mixedema).
Hyperthyroidism
Hyperthyroidism (thyrotoxicosis) is a hypermetabolic clinical and biochemical state by
excess production of thyroid hormones.
Many diseases may cause hyperthyroidism, but three most common causes are:
1. Graves’ disease (diffuse toxic goitre).
2. Toxic multinodular goitre.
3. Toxic adenoma.
Less frequent causes are thyroiditis, metastatic tomors of thyroid, struma ovarii, congenital
hyperthyroidism
Goitre
Goitre is defined as thyroid enlargement caused by compensatory hyperplasia and hypertrophy
of the follicular epithelium is response to thyroid hormone deficiency.
Goitre is classified according to their morphology and epidemiology, course, functional and
clinical peculiarities.
I. According to the morphology goitre may be:
1. Simple goitre (diffuse nontoxic or colloid goitre).
2. Nodular goitre (multinodular goitre or adenomatous goitre).
3. Diffuse nodular (mixed).
II. According to the histology there are 2 types of goitre:
146
1. Colloid. Colloid goitre may be macrofollicular and microfollicular as well as mixed type. It
consists of follicles. In case of epithelial proliferation the disease is termed proliferating colloid
goitre, which is usually nodular.
2. Parenchymal. Parenchymal goitre is characterized by epithelium proliferation with formation
of small follicle-like structures without colloid. In the majority of cases the disease is diffuse.
III. According to the epidemiology goiter is classified into:
1. Endemic. Endemic goitre develops in the areas with iodine deficiency in the drinking water
(the Urals, Siberia, Middle Asia, Switzerland). The thyroid gland has the structure of colloid or
parenchymal goitre. The functional activity is decreased. In children, endemic cretinism may
develop (physical and mental retardation).
2. Sporadic. Sporadic goitre manifests in young and old age. This may be colloid; diffuse or
mixed. It does not influence the organism as a whole, but it can cause compression of the
esophagus, trachea, larynx, etc. with disturbance of their function. This goitre may be the
cause of Basedow’s disease.
Graves’ disease
Graves’ disease (or diffuse toxic goiter, Basedow’s disease, primary hyperplasia,
exophthalmic goitre)
Diffuse toxic goiter is an autoimmune disease.
Morphology: prismatic epithelium turns into cylindrical, epithelium proliferation with
formation of papillae, colloid vacuolization, lymphoid plasmocytic infiltration of the
stroma, formation of lymphoid follicles with germ centers are observed.
In the other organs, hypertrophy of the left ventricle of the heart, serous edema and
lymphacytic infiltration myocardial interstitial spaces develop (thyrotoxic heart). The
outcome is diffuse interstitial sclerosis. In the liver, there is serous edema causing
thyrotoxic liver fibrosis. Thymus enlargement causes lymphoid tissue hyperplasia and
adrenal hypertrophy. Exophthalmus takes place.
The causes of death are cardiac insufficiency and cachexia.
Hypothyroidism (mixedema)
Hypothyroidism is a hypometabolic clinical state resulting from inadequate production of
thyroid hormones of prolonged periods of, or rarely, from resistance of the periferal tissues tj the
effects of thyroid hormones. The clinival manifestations of hyperthyroidism are divided to into group:
I. Cretinism or congenital hyperthyroidism.
It produces the clinical syndrome, which occurs a puffy face and enlarged tongue (coarse
features), a protuberant abdomen, and delayed physical and mental developmental milestones. The
main causes of cretinism are:
Untreated maternal hypothyroidism. This is now rare, due to better prevention,
recognition and treatment of maternal hypothyroidism but it is still a problem in some
areas of the world where endemic goiter due to dietary iodine deficiency is seen.
Inherited enzyme defect. This produces sporadic cretinism and is due to failure of normal
T3 and T4 synthesis.
II. Myxedema
It is the adult hypothyroidism, which is due to reduced metabolic rate. The term “myxedema”
connotes non-pitting edema due to accumulation of hydrophilic micopolysaccharides in the ground
substence of dermis and others tissues. There is progressive slowing of physical and mental activity,
increasing lethargy and sensitivity to cold, puffy face, coarse dry skin, thinning of hair (particularly of
the eyebrows), hoarseness and deepening of voice, and various internal abnormalities, particularly
heart failure and a predisposition to hyperlipidemia and hypothermic coma.
The main causes of myxedema are:
Surgical ablation of the thyroid gland, which is usually as a result of total thyroidectomy
for malignant disease, or aggressive subtotal thyroidectomy for hypothyroid Graves’
disease.
Hashimoto’s thyroiditis.
Some drug e.g. lithium.
147
Thyroiditis
Thyroiditis is classified into the following types:
1. Hashimoto’s thyroiditis.
2. Infectious thyroiditis.
3. Granulomatous thyroiditis (de Quervain’s thyroiditis or giant cell thyroiditis).
4. Riedel’s thyroiditis (or invasive fibrous thyroiditis, Riedel’ struma).
Hashimoto’s thyroiditis
Hashimoto’s thyroiditis is a destructive autoimmune thyroiditis leading to
hypothyroidism.
It is most common in middle age, affecting women more often than men, a good example
of organ-specific autoimmune disease.
The following autoantibodies against different thyroid cell antigens are detectable in the
sera of most patients:
1. Thyroid microsomal thyroiditis.
2.m Thyroglobulin autoantibodies.
Morphology
Two varieties of Hashimoto’s thyroiditis are seen: classic from (more common), and
fibrosing variant (only 10% cases).
Macroscopically, the classic form is characterised by diffuse, symmetric, firm and rubbery
enlargement of the thyroid which may weigh 100-300 gm. Sectioned surface is fleshly
with accentuation of normal lobulations but with retained normal shape.
Microscopically, the classic form shows the following features:
There is extensive infiltration of the gland by lymphocytes, plasma cells,
immunoblasts and macrophages, with formation of lymphoid follicles having
germinal centres.
There is decreased number of thyroid follicles, which are generally atrophic and
are often devoid of colloid.
The follicular epithelial cells are transformed into their degenerated state
termed Hurthle cells (Askanazy cells). These cells have abundant oxyphilic or
eosinophilic and granular cytoplasm due to large number of mitochondria and may
cantain large nuclei.
There is slight fibrous thickening of the septa separating the thyroid lobules.
Hashimoto thyroids proceed to primary atrophic thyroiditis or lead to carcinoma.
Riedel’s thyroiditis
Riedel’s thyroiditis is characterised by stony-hard thyroid that is densenly adherent to the
adjacent structures in the neck.
The etiology is inknown.
Macroscopically, the thyroid gland is contracted, stony-hard, assemetric and adherent to
the adjasent structures. Cut section is hard and devoid of lobulations.
Microscopically: extensive fibrocollagenous replacement, atrophy of thyroid tissue, locally
scattered lymphocytic infiltration and invasion of the adjusent muscle by the process.
Diabetes mellitus
Diabetes mellitus is a chronic clinical syndrome characterised by hyperglycemia due to
deficiency or defective response to insulin.
This is classified into:
1. Spontaneous diabetes mellitus is an independent disease and can be of 2 types:
Type I (insulin- dependent).
Type 2 (insulin-independent).
2. Secondary diabetes may occur in pancreatic diseases, acromegaly, Itsenko-Cushing disease, and
complicated genetic syndromes, at administration of some “drugs”.
148
3. Diabetes of pregnant occurs during pregnancy.
4. Latent (subclinical) diabetes is not evident.
Etiopathogenetic factors:
Genetically determined disturbances of the number and structure of beta-cells.
Environmental factors, which disturb beta-cell nutrition (bacteria, viruses, autoimmune
reactions), increase of activity of adrenergetic nervous system.
Risk factors of different kinds of spontaneous diabetes are different.
Pathogenesis
Insulin insufficiency increases blood glucose amount because cellular membranes are closed
for glucose - hyperglycemia and glucosuria develop. Considerable amount of sugar is formed from the
fats and proteins causing hyperlipidaemia, aceton- and ketonemia.
Morphology
The pancreas is diminished with lipomatosis and sclerosis.
Degeneration and hyalinosis are observed in the islets, some of them are hypertrophic.
The liver is enlarged, glycogen is absent, fat degeneration is observed.
Diabetic macro- and microangiopathy is seen in the vessels.
Macroangiopathy is arterial atherosclerosis.
Microangiopathy is characterized by plasmatic saturation, hyalinosis, and sclerosis with
lipohyalin.
Vasculitis takes place.
There is generalized microangiopathy in the kidneys, retina, skeletal muscles, digestive
tract mucosa, pancreas, brain, and nerves.
In the kidneys, diabetic glomerulonephritis and glomerulosclerosis develop.
Microscopically proliferation of mesangial cells in response to mesangium clogging with
“ballast” metabolic products and immune complexes are observed. Mesangium hyalinosis
and glomerulosclerosis take place.
Diabetic glomerulosclerosis may be diffuse and nodular as well as mixed type. Its clinical
manifestations are Kimmelstiel-Wilson syndrome (proteinuria, edema, increased arterial
pressure).
In the lungs, lipogranulomas consisting of macrophages and gigantic cell of foreign
bodies are present in the walls of the arteries.
In the spleen, liver, lymphatic glands: infiltration of brstiomacrophagal system and skin
with cell lipids (xantomatosis) develop.
Complications:
Diabetic coma.
Accompanied with macroangiopathy: gangrene of extremities, myocardial infarction.
Diabetic nephropathy (acute and chronic renal failure).
Diabetic retinopathy is a leading cause of blidness.
Infectious sepsis.
The death is caused by coma, diabetic glomerulosclerosis, gangrene.
149
PRENATAL PATHOLOGY
The period of fetus development beginning with the moment of fertilization to the birth of
the child is called Prenatal period.
Duration of the prenatal period is 40 weeks (280 days) or 10 lunar months or 9 calendar’s
months.
Fetal pathology, which occurs in this period, is called prenatal pathology.
The case of fetal death before the 14th week of gestation is called abortion that within the
period of 14-22 weeks is called late abortion. If the fetus dies on the 22nd week or later
(until the delivery or during it), the case is called mortinatality.
The normal and pathologic development and growth can be divided into the following
stages:
1. Progenesis is characterised by gametogenesis, i.e.formation and maturation of the gametes. It stage
occurs before the fertilization of the ovum. Pathology of gametogenesis is called gametopathy.
2. Development after fertilization is called kymatogenesis. This intrauterine phase can be divided
into:
a) Blastogenesis from Day 1 to Day 15 of gestation. Pathology of blastogenesis is called
blastopathy.
b) Embryogenesis from Day 16 to the end of the 3rd month (75th day). Pathology of
embryogenesis is called embryopathy.
c) Fetogenesis from the 4rd month of gestation to delivery (from 76th to 280th day).
Pathology of fetogenesis is called fetopathy.
Gametopathy
Gametopathy is an injury of formation and maturation of the gametes during ovo- and
spermatogenesis until fertilization.
Gametopathy occurs in gene mutations and chromosomal aberrations. At present about
150 autosomal recessive genetic defects and 200 defects with autosomal dominant
inheritance are known. There are also defects connected with sex X chromosome.
Chromosome mutations are called chromosomal aberrations. Virtually all the
chromosomal syndromes are characterized by congenital anomalies.
Genetic injuries in origin can be deviled into three groups:
a) Those associated with karyotypic aberrations.
b) These arising in single gene mutations.
c) Those suspected of resulting from multifactorial inheritance, a term that implies the
interaction of two or more genes of small effect with environmental factors.
The most frequent is trisomy 21 (Down syndrome) and trisomy 13 (Patau’s syndrome),
trisomy 18 (Edward’s syndrome), Klinefelter’s syndrome, Turner’s syndrome.
Down’s syndrome
Down syndrome is the most common of the chromosomal disorders and a major cause
of mental retardation.
The risk of the development of Down syndrome increases with maternal age.
It is a disorder associated with autosomes.
Trisomy 21 type – 47XXC2 or 47XXYC1. The majority of cases of Down’s syndrome are due
to nondisjunction of maternal meiosis.
Currently more than 80% survive to age 30 or beyond.
The most causes of death are interrcurrent infections or cardiac insufficiency.
Gross appearance:
Short stature.
Muscle hypotonia.
Hyperflexibility of joints and lack of Maro reflex.
Short crooked fifth finger.
Short broad hands with a single simian crease on the palm.
Flat facial profile.
Low-bridged nose.
150
Dysplastic ears.
Reduced interpupillary distance.
Oblique palpebral fissures.
Epicanthic folds.
Brushfield spots (the iris may be speckled).
The mouth is often open and protruding tongue.
Clinical significances:
Mental retardation is usually severe.
Low intelligence.
May be leukemia.
Patients with Down syndrome have abnormal immune responses that
predispose them to serious infections, particularly of the lungs.
Virtually all patients with trisomy 21 older than 40 years of age develop
neutropathologic changes characteristic of Alzheimer disease, a form of senile
dementia.
Thyroid dysfunction: hyperthyroidism, goiter, and hypothyroidism.
Congenital heart defects.
Gastrointestinal anomalies.
Edward’s syndrome
Trisomy 18E – 47XXE or 47XYE.
May die in the neonatal period, and the majority do not survive beyong 1 year.
Survivors have severe mental retardation and failure to thrive.
Characteristics:
Hypertonicity.
Prominent occiput.
Micrognathia and low-set ears.
Flexion of fingers (index over third).
Short sternum and small pelvis.
Abnormalities of the hips and feet (syndactyly), rocker-bottom feet.
Cardiac defects: patent, ductus arteriosus and interventricular septal defects.
Renal malformations.
Meckel’s diverticulum.
Absence of the corpus callosum and incomplete development of the cerebellum.
Patau’s syndrome
Trisomy 13D – 47XXD or 47XYD occurs at frequency of 1 in 20000 births.
Most children die in the first month, and those who survive have severe mental
retardation.
Characteristics:
Microphaly and archinencephaly.
Scalp defect.
Coloboma of the iris.
Microphtalmos.
Anophtalmos.
Cleft palate.
Hair lip.
Polydactyly.
Hemangiomas of the head.
Neck and lower back.
Rocker-bottom feet.
Apneic spells and myoclinic seizures.
Cardiac dextraposition and interventricular septal defect.
Extensive visceral defects: polycystic of kidneys, ectopia of spleen into
pancreas; double uterus and vagina.
Turner’s syndrome
It is gonadal dysgenesis (defective second X chromosome – 45 X0).
Somatic anomalies:
Short stature.
151
Primary amenorrhea.
Webbing of the neck.
Cubitus valgus (an increase in carrying angle of the arm).
Shield – like chest with widely spaced nipples.
Coarctation of the aorta.
Webbing of the digits or of the axillae.
Senile facies.
High-arched palate.
Low-set ears.
Peripheral lymphedema at birth.
Pigmented nevi.
Low posterior hairline.
Uterus, ovarium and fallopian tubes are infantile.
Secondary sex characteristics are absent.
Characteristic laboratory and morphologic findings are:
Negative sex chromatin test.
Ovaries are replaced by white “streaks” of fibrous stroma devoid of follicles.
Reduced levels of ovarian estrogenes.
Increased gonadotropin.
Clinical significans:
It is an important cause of sterility in the female.
Normal intelligence.
Blastopathy
Blastopathies occur during the first 15 days from the moment of fertilization.
The most frequent cause of blastopathy is chromosomal aberration in combination with
harmful effect of environmental factors.
Manifestations of blastopathies are different:
a) Superficial or deep implantation of blastocyst (causes defects of development, shape,
localization of placenta).
b) Disturbance of embryo orientation (umbilical-cord defects are the most frequent).
c) Empty embryo sacs (blastocytes without an embryo).
d) Double malformations are reduplicated embrional primordial that are either
primary or the result of later devision. They may be independent of each other, i.e. they
may be connecting only by the placenta or the umbilical cord, or they may be in direct
bodily contact. Usually they cannot live.
Double malformations may be free and conjoined:
I. Free double malformations are designated as twins (gemini). They may be identical and fully
developed (monosigotes twins), or they may be malformed. Holocardius Acephalus is the most
frequent of the free double malformations in which only the trunk and lower extremities are clearly
identifiable, while the head is absent.
II. Conjoined double malformations occur:
1. Asymmetric (parasitic) or heteropagus (one of the twins is underdeveloped).
Asymmetrical double monsters have one well developed and one rudimentary or
hypoplastic twin. The rudimentary twin is always abnormal, and is either externally
attached to or internally included in the body of the better-developed sibling (fetus in
fetu). Some of the congenital teratomas, especially those in the sacrococcygeal area, are
actually asymmetrical monsters. Teratomas are regarded as asymmetric monsters.
2. Symmetric forms or diplopagus represent two equally well developed embrional
primordial that are connected to each other by a partial fusion of tissues. They may be:
a) Incomplete individuals result from extensive fusion i.e., there is only an
incomplete reduplication of the body axis. This type of malformation includes
the dicephalus (to spinal columns but only one pelvis).
b) Complete symmetrical double malformations show a partial fusion between
two generally mature fetuses. The important feature is the reduplication of the
body axis (head, trunk, or spinal column). It is called cephalopagus, diprosopus
(reduplication of the face), craniopagus, thoracopagus, and ischiopagus
(“Siamese twins”).
Embryopathy
152
Embryopathy is a pathology developed within the period of 16 - 75 days and occurs by the
development of the congenital malformations.
Congenital malformations are morphologic defects that are present at birth, although
they may not become clinically apparent until later in life. The term congenital does not
imply or exclude a genetic basis for malformations. It is estimated that about 3% of
newborns have a major malformation, defined as a malformation having either cosmetic
or functional significance.
Pathologic development is connected with the termination period in which the causative
agent acts. Each organ has its own period of teratogenic factor action. This period is
called teratogenic termination period.
Causes of the congenital malformations
I. Environmental influences, such as viral infections, drugs, and irradiation, to which the mother was
exposed during pregnancy, may induce malformations in the fetus and infant.
TORCH infections are caused by Toxoplasma (T), rubella (R), cytomegalovirus (C),
herpes virus (H), and a number of other (O) bacterial and viral agents. The latter include
fever, encephalitis, chorioretinitis, hemolytic anemia, hepatosplenomegaly, pneumonitis,
myocarditis, and vesicular/hemorrhagic skin lesions. Such infections, occurring early in
gestation may also cause chronic sequelae in the child, including growth and mental
retardation, cataracts, congenital cardiac anomalies, and bone defects.
A variety of drugs and chemicals have been suspected to be teratogenic. The list includes
thalidomide, folate antagonists, androgenic hormones, alcohol, anticonvulsants, warrafin
(oral anticoagulant).
Radiation.
II. Genetic factors.
Any congenital defect may manifest as one of the following changes:
Agenesis is the complete absence of an organ pri-mordium.
Aplasia is absence of the organ coupled with persistence of the organ anlage or a
rudiment that never developed completely.
Hypoplasia refers to reduce size due to the incomplete development of the organ.
Dysraphic anomalies are defects caused by failure to fuse. Spina bifida is an anomaly
in which the spinal canal has not closed completely and the overlaying bone and skin have
not fused, thus leaving a midline defect.
Involution failures are defects due to the persistence of embryonic or fetal structures
that should involute at certain stages of development.
Division failures are defects caused by incomplete cleavage, when that process depends
on the involution and programmed death of cells. Fingers and toes are formed at the
distal end of the limb bud through programmed death of cells between the primordia that
contain the cartilage. If these cells do not die in a predetermined manner, the fingers will
be conjoined or incompletely separated (“syndactyly”).
Atresia refers to defects caused by incomplete formation of a lumen. Many hollow
organs originate as strands and cords of cells, the centers of which are programmed to
die, thus forming a central cavity or lumen. Atresia of the esophagus is characterized by
partial occlusion of the lumen, which was not fully established in embryogenesis.
Dysplasia is a defect caused by abnormal organization of cells into tissues, a situation
that results in abnormal histogenesis.
Ectopia or heterotopia is an anomaly in which an organ is outside its normal
anatomical site. Thus, ectopic heart is located outside the thorax. Heterotopic parathyroid
glands can be located within the thymus in the anterior mediastinum.
Classification of congenital defects
1. According to the character of involvement.
Isolated (one organ).
Systemic (several organs of one system).
Multiple (in different organs and systems).
2. According to localization.
Central nervous system.
Cardiovascular system.
Alimentary tract.
Urinary system, etc.
153
Central nervous system, and cardiovascular system are most frequently involved because these
systems have the longest teratogenic terminal period, from the 18th day to the 50th day.
Malformations of the nervous system
I. Errors of fusion
1. Craniorachischisis totalis due to failure closure of the neural tube the convexity of the skull is
absent and the spine is represented only by it’s bodies with no posterior covering.
2. Spina bifida occulta due to the failure of closure of the sacral bones (rachischisis):
a) Meningocele, diverticulum – like bulging of an arachoid sac filled with clear spinal
fluid.
b) Meningomyelocele in which the arachnoidal sac also contains parts of the spinal
cord, the cauda equna or the area medullovasculosa (see above), which protrudes
because of the accumulation of fluid.
c) Meningomyelycystacele or syrengomyelocele, a combination of meningocele and
hydromyelia (ballooning of the spinal cord due to a hydrophic accumulation of the
fluid in the central canal).
3. Anencephally is an absence of the cranial vault (acrania) and of the brain (anencephaly) and a
short neck.
4. Microcephaly is decrease of the brain’s size.
5. Amyelia, or total aplasia of the spinal cord.
6. Diplomyelia (Each half of the spinal cord develops separately over many segments).
7. Diastematomyelia (each half has only one dorsal and one ventral horn with a intervening
cystic cavity to represent the central canal).
8. Arnold-Chiari malformation is a group of different combinations (the full deformity is a
herniation of the posterior cerebellum, the medulla and the foramen magnum, with an added
sharp curvature of the neuraxix at the cervicomedullary junction).
9. Congenital stenosis of the aqueduct.
10. Cyclopia (one eye in the middle of a deformed forehead).
11. Arrhinencephaly (the olfactory buld and tracts are absent in the mildest form of the disorder).
12. Agenesis of the corpus callosum is a component of arrhinencephaly.
13. Hydrocephalus is meant an increased amount of cerebrospinal fluid in the
ventriculosubarachnoid pathways of the brain.
II. Errors of migration (Heterotopies, Ectopias)
1. Status verrucosus is a wart-like appearance of the cortex produced by a disorderly
arrangement of the neuroblasts so that fissuration and sulcation are irregular and
unpredictable.
2. Pachygyria is the appearance of large gyri in the cortex due to inadequate differentiation of
sulci.
3. Microgyria (secondary fissures are lacking).
Congenital malformations of the heart
Congenital malformations of the heart represent structural changes that originate during
the development of the septa and the rotation of the arterial heart, during the first 3
months of gestation.
Congenital heart defects are the most common forms of heart disease in children. They
occur in about 1% of neonates and in 18% of spontaneous aborted and stillborn fetuses.
Most defects manifest themselves within the first year of life, such as large ventricular
septal defects and tetralogy of Fallot.
Others, like bicuspid aortic valve, remain silent until middle age, when degenerative
changes of the abnormal valve cause stenosis.
Still other defects, like a small ventrical septal defects (VSD) or patent foramen ovale may
never cause any difficulty at all.
The most important malformations are:
I. Septal defects
154
Patent foramen oval (Atrial septal defect).
Ventricular septal defects (Roger’s disease).
Complete absence of the ventricular septum, with or without septal defect (cor bilocular or
cor triboculare).
II. Transposition defects
Dextraposition of the aorta (Eisenmenger’s syndrome, tetralogy Fallot).
Dextraposition of the heart.
III. Stenoses
Tricuspid stenosis or atresia.
Pulmonary stenosis.
Mitral stenosis or atresia.
Aortic stenosis or atresia.
Aortic coarctation.
IV. Valvular insufficiency
Congenital tricuspid insufficiency (or Ebstein’s anomaly).
Congenital mitral insufficiency.
V. Persistent fetal vessels
Patent ductus arteriosus (with many congenital cardiac defects).
Anomalies of the pulmonary veins include openings into the superior or inferior vena cava
or the coronary sinus.
VI. Combined heart defects
A. Trialogy of Fallot consists of
1. Ventricular septal defect.
2. Stenosis or atresia of the pulmonary outflow tract.
3. Right ventricular hypertrophy.
B. Tetralogy of Fallot consists of
1. Stenosis or atresia of the pulmonary outflow tract.
2. Ventricular septal defect.
3. Aorta overriding the right ventricle (aorta dexraposition).
4. Right ventricular hypertrophy.
C. Pentalogy of Fallot consists of
1. Ventricular septal defect.
2. Stenosis or atresia of the pulmonary outflow tract.
3. Hypertrophy of the right heart.
4. Aorta dexraposition.
5. Defect of interatrial septum.
Classification anomalous development of the heart (by Ebbott)
I. Acyanotic shunt (left-right)
1) Patent ductus arteriosus.
2) Atrial septal defect.
3) Ventricular septal defect (Roger’s disease).
II. Cyanotic shunt (Right-left)
1) Tetralogy of Fallot.
2) Eisenmenger’s complex (variant of the tetralogy of Fallot).
3) Transposition of great vessels.
III. No shunt
1) Coarctation of the aorta.
2) Aortic stenosis.
Examplies of some heart defects
Patent ductus arteriosus (PDA)
Before birth the ductus arteriosus (DA) is the only route by which blood in the right
ventricle (RV) can reach the systemic circulation.
155
The DA is located superior to the bifurcation of the pulmonary arteries and enters the
aorta just distal to the left subclavian artery. It is invested with spirals of smooth muscle,
which contract at birth in response to high tension (coming from the newly aerated
lungs). Within a day or two, the DA is usually functionally closed.
Not infrequently and often in premature infants, the DA fails to close, hence a PDA. With
the drop in pulmonary arterial pressure, which occurs following lung inflation after birth,
blood shunts from the aorta across the PDA and into the main pulmonary artery.
The RV responds to increased flow and pressure with either dilatation (congestive heart
failure) or hypertrophy.
If pulmonary resistance exceeds systemic resistance (due to hypoplastic lungs, for
instance), blood is shunted right-to-left and cyanosis may ensue.
Atrial septal defects (ASD)
The most common ASD is a secundum ASD or ASD II. A secundum ASD means that the
ASD is reminiscent of the ostium secundum i.e., the ostium secundum is not covered over.
An ASD II may form in any of four ways:
1. Deficient formation of septum primum.
2. Deficient formation of septum secundum.
3. Combination of both.
4. Dilation of the atria, thereby pulling septum primum and septum secundum apart.
After birth, the blood shunts from left to right atrium. This shunt may be large if the septal
defect is large.
In atrial septal defects, the pulmonary artery pressure falls to normal or near normal
levels soon after birth. However, the resulting atrophy of the right ventricle makes this
chamber more distensible than the left ventricle.
With a sizable atrial defect, pressures in the two atria are similar and the right ventricle
fills more easily than the left.
Consequently, not only does the vena caval blood enter the right ventricle but also much of
the pulmonary venous blood from the left atrium enters the right ventricle as well.
Pulmonary blood flow is greatly increased while oxyhemoglobin saturation in the
systemic arterial system is normal.
The pulmonary arterial bed responds to this “over circulation” with smooth muscle
hyperplasia and hypertrophy.
If the septal defect is operatively closed at this stage, the pulmonary arterial muscle will
atrophy and a normal circulatory pattern will be established. If a large defect is not closed
in time, diastolic overloading of the right ventricle continues and both the right ventricle
and atrium dilate and hypertrophy.
Left ventricular output is usually near normal so that these infants develop normally.
Over the course of several decades, pulmonary vascular obstruction takes place within the
pulmonary arterial bed.
This progressively narrowed bed offers greater and greater resistance to pulmonary
blood flow. Resistance in the pulmonary arterial bed may eventually equal or exceed that
in the systemic bed.
When this occurs, shunts are reversed and cyanosis appears or becomes more prominent.
If the normal cardiac anatomy is now restored through surgery, such patients will
develop right-sided cardiac failure since the right ventricle is incapable of forcing all of
the caval blood through the restricted pulmonary vascular bed.
Ventricular septal defects (VSD)
These are the most common of the cardiac defects. The ventricles are separated by the
growth of the muscular septum and by the growth and fusion of the endocardial cushions
near the atria. VSD’s can be located near the atrioventricular valves or nearer to the apex
(i.e. tip) of the heart.
Before birth, VSD’s are of little consequence since the pulmonary arterial pressure is equal
to the systemic pressure and thus, most of the blood enters the systemic circulation.
After birth, VSD’s may be of little consequence if they are small. But, if they are large, they
allow significant left-to-right shunting, since now (after birth) the pulmonary (right)
pressure is lower than the systemic (left) pressure.
156
Thus, the pulmonary vascular bed is faced with abnormally high pressures, as is the RV.
The pulmonary vessels become thicker by undergoing smooth muscle
hyperplasia/hypertrophy.
The RV may also hypertrophy. These changes reflect the presence of pulmonary
hypertension.
Because of this left-to-right shunting, pulmonary blood flow may be greatly increased,
leading to pulmonary congestion, edema, hemorrhage, pneumonia and congestive heart
failure.
After a long time, pulmonary pressure may equal or even exceed systemic resistance. In
the latter situation, blood shunts from right-to-left, resulting in cyanosis.
Common atrioventricular canal (CAVC)
In this cardiac malformation, the endocardial cushions fail to separate the
atrioventricular canal into right and left sides.
Because the atrioventricular valves (mitral and tricuspid) are also formed by the
endocardial cushions, they are malformed as well.
Thus, blood from the right atrium (RA) and the left atrium (LA) enters into a common,
undivided atrioventrical (AV) canal above the incompletely divided RV and left ventricle
(LV). Much mixing of blood occurs.
This defect is commonly associated with Down’s syndrome (Trisomy 21).
Transposition of the great arteries (TGA)
This is a fairly common defect and it is important to recognize it early since surgical
correction is possible and successful.
It is thought to be due to abnormal development of the conal muscle beneath the two
semilunar valves.
In this defect the aorta arises from the right ventricle and the pulmonary artery arises
from the left ventricle. In utero, this defect causes no trouble, since venous blood (from the
placenta) is oxygenated.
After birth, however, the pulmonary and systemic circulations are disconnected.
Venous blood enters the RA, then the RV and then out the aorta. Pulmonary venous blood
enters the LA, then the LV and then out the pulmonary arteries back to the lungs.
Survival is dependent on any kind of mixing of oxygenated and unoxygenated blood
whether through an ASD, a VSD or a patent ductus arteriosus.
Whatever mixing of the two systems there is, it is usually inadequate and these neonates
have marked cyanosis.
If the communication between the two circulations is adequate for oxygenation, then
problems of cardiac failure and pulmonary hypertension will inevitably develop.
Combined heart defects:
Tetralogy of Fallot
It was Etienne-Louis Arthur Fallot in 1888 who well described this form of cyanotic heart
disease. Fallot did not actually coin the term, “tetralogy of Fallot”. He used the term la
maladie bleu, i.e., the blue disease. It was Maude E. Abbott who first used this term
tetralogy of Fallot in 1924.
What comprises tetralogy of Fallot?
1. Stenosis or atresia of the pulmonary outflow tract.
2. Ventricular septal defect.
3. Aorta overriding the right ventricle.
4. Right ventricular hypertrophy.
Actually, the basic abnormality is underdevelopment of the subpulmonary cone of muscle
(conus), resulting in stenosis/atresia of the pulmonary outflow tract.
This underdevelopment of the conus results in malaligament of a band of muscle (the
parietal band) that lies beneath the pulmonic valve.
In tetralogy, this band of muscle shifts anteriorly, superiorly and to the left, which
obstructs the pulmonary outflow tract. This opens up a “hole” in the interventricular
septum (hence a VSD).
With this leftward shift, the aorta is “looking down into” the RV (hence an “overriding”
aorta).
157
Finally, with the narrowed pulmonary outflow tract, there is obstruction (hence right
ventricular hypertrophy).
The VSD also contributes to right ventricular hypertrophy.
So, in essence, Fallot’s congenital anomaly could be called “monology of Fallot” with the
single underlying abnormality being an underdeveloped conus resulting in a stenotic or
atretic pulmonary outflow tract. The other three abnormalities are a result of this one
abnormality.
The degree of cyanosis depends on the degree of pulmonary outflow obstruction. This
obstruction can be at the level of the pulmonic valve or in the right ventricle just beneath
the pulmonic valve.
The right ventricle hypertrophies, in response to the increased resistance and because of
the large VSD. With severe right ventricular outflow obstruction, right to-left shunt occurs
across the VSD and the patient suffers from severe cyanosis.
Surgical repair is now highly effective; complete repair usually being done within the first
two years of life.
Despite the nearly unbelievable variety of cardiac malformations that are possible, the net
result of these abnormalities can be reduced to a mercifully short list of clinical signs and
symptoms.
Congenital anomalies of the kidneys and the lower urinary tract
Agenesis.
Hypoplasia.
Displacement.
Polycystic kidney disease.
Horseshoe kidney.
Accessory (Additional) kidneys.
Reduplication of the urachus.
Congenital anomalies of the genital system
I. Male
Phimosis.
Hipospadias and epispadias.
Cryptorchidism.
Septate uterus.
Double uterus with double cervix.
II. Female
Congenital anomalies of the gastrointestinal tract
Agenesis (esophagus, intestinum).
Atresia.
Fistulous tract of the esophagus with the trachea or main stem bronchi.
Stenosis.
Megaesophagus.
Diaphragmatic Hernias.
Pyloric stenosis.
Meckel’s Diverticulum.
Megacolon (dilation of bowel) in Hirschprung’s disease. Hirschsprung’s disease results
from a failure of migration of neuroblasts that form the myenteric plexus. In all patients,
ganglioncells are absent in the region of the anorectal junction. The result is intestinal
obstruction in affected neonates. The incidence is 1 per 5000 live births.
Congenital Cystic liver disease.
Agenesis, hypoplasia, hyperplasia, total reduplication of the qallbladder.
Ectopia of the pancreas.
Congenital anomalies in the face (8th to 10th week of the embryonal period)
1. Lateral facial clefts:
a) Cheiloschisis or cleft lip (limited to the upper lip).
b) Gnathoschisis (extend to the maxilla).
c) Palatoschisis (extend to the hard palate).
158
2. Median facial clefts may also be limited to the upper lip, maxilla, or palate. The nose is troguently
flat due to the aplasia of the vomer.
3. Oblique facial clefts extend from the upper lip to the corner of the eye.
Congenital malformations of the bone-cartilage system
Hondrodysplasias is characterized by the shortening and thickening of the legs.
Achondroplasia is a derangement in epiphyseal cartilaginous growth resulting in
dwarfism.
Hondrodysplasia of fetus (lethal micromelia):
a) Chondrodysplasia.
b) Increase of the head.
c) Open mouth.
d) Saddle-like nose.
e) Thickening of the tongue.
f) Short neck.
g) Hypoplasia of the thorax and lungs.
h) Thickening of vertebra.
Sirenomelia. The term comes from “siren” or “mermaid” because of the characteristic
fusion of the lower extremities that results from a failure of normal vascular supply from
the lower aorta in utero.
Aplasia (Amelia) of legs.
Phocomelia is underdevelopment of the proximal parts of legs, when the foot and arm
growth from trunk.
Polyductyly is a malformation consisting of supernumerary finger development.
Syndactyly is partial or complete fusion of several fingers.
Defects of placental development
Placental hypoplasia (normal mass is 0.5 - 0.7 kg), placenta/fetus ratio is 1/5 -1/7. When
the mass of the placenta decreases, fetus hypoplasia develops.
Defects of placenta localization are marginal and central placenta presentation in respect
to internal uterus orifice. This develops as a result of Mastopathy, its causes are unknown,
and it presents a risk of placenta detachment during the delivery and intranatal death of
the fetus.
Defects of placenta detachment are placenta adhesion (caused by deep implantation of
blastocyst). The placenta does not detach after the delivery. Hemorrhage may occur.
Placenta abruption. This occurs at extragenital and genital maternal pathology. It may
result in intranatal fetus asphyxia.
Umbilical cord defects (its normal length is 0.5 - 0.7 m). If the length is less than 0.5 m, the
cord is short, more than 0.7 in it is long.
Disturbance of the umbilical cord attachment to the placenta:
1. Central and eccentric are normal types of attachment.
2. Membranous is pathological one, when the umbilical cord is attached to the
membranes, its vessels may be compressed with the parts of the fetus and amniotic
fluid, which may cause their rupture, ante-, and intranatal fetus death may occur.
Amnion development defects: - hydramnion (2000 ml and more), oligoamnios (500 ml
and less).
Fetopathy
Fetopathy is characterised by the combination of the impairment of tissue morphogenesis
and reactive processes.
In early period the impairment of tissue morphogenesis predominates.
In late period the reactive processes such as the disturbance of the blood circulation,
degenerations, necrosis, inflammation, presence of immune reactions, compensative and
adaptative processes, regeneration.
Fetopathies may be infectious and non-infectious.
Infectious fetopathies
Infectious fetopathies can be connected with the influence of viruses and bacterium.
Inflammation in placenta takes place.
159
Pathologic morphology of fetopaties:
a) Necrosis of parenhymatous organs and brain (rubella, cytomegaly, chickenpox,
toxoplasmolis).
b) Formation of granulomas (syphilis, tuberculosis).
c) Hemorrhagic syndrome.
d) Immune reactions.
e) Hepato- and splenomegaly.
f) Jaundice.
The main infectious fetopathies are cytomegaly and congenital toxoplasmosis.
Cytomegaly
Cytomegaly (from cytos - cell and megaios - large) is a virus infection involving
salivary glands. The disease is characterized by formation of giant cells with intranuclear
inclusions.
Generalized form of infection develops in the newborns. DNA-containing virus enters the
organism of the fetus from the mother through the placenta.
Generalized infection in children is characterized by central nervous system (CNS)
involvement, which is not observed in acquired cytomegaly. Encephalitis with formation
of cytomegalic cells, perivascular infiltration and calcinosis foci in the subependymal zone
are observed in children. These phenomena cause hydrocephalia.
Cytomegalic cells contain intranuclear formation resembling “owl’s eye”. They can be
found in the lungs, kidneys, liver, intestine, pancreas, adrenal gland, and thymus.
Hemorrhages and necroses can also be observed in these organs.
The disease lasts several days (sometimes weeks). It ends with death caused by damage to
vitally important organs.
Congenital toxoplasmosis
Congenital toxoplasmosis is a disease caused by toxoplasma. It develops as a result of
hematogenic transfer from the mother’s organism.
Toxoplasma is a protozoic microorganism from tripanosomid family. The source of
human infection is dogs and cats. The fetuses are infected through the maternal placenta.
During teratogenic termination period, embryopathy incompatible with life occurs.
Early period is characterised by microcephalia of brain, porencephalia with gliosis
(consolidation of the remained brain tissue) and calcinosis. Microscopic examination
demonstrates cysts filled with granular spheres.
Late period occurs the foci of necrosis, and calcinosis in the brain, pseudocysts and free
parasites, encephalitis in the whole brain, meningopathy, ependymatitis, and
hydrocephalus. Productive necrotic rhinitis and uveitis in the retina. Generalized form:
besides GNS involvement there is hepato- and splenomegaly, jaundice, ulcers of the
intestine, myocarditis, interstitial pneumonia. Microscopic examination demonstrates
erythroblastosis in the liver and spleen, necrosis, calcinosis and lymphohistiocytic
infiltration in the liver, myocardium, kidneys, cholestearosis in the liver.
Outcome: death of fetuses and newborns or complication (paralysis, mental retardation,
hemorrhage).
Noninfectious fetopathies
The main noninfectious fetopathies are hemolytic disease of the newborn, fetal
mucoviscidosis, fibroelastosis of myocardium and diabetic fetopathy.
Early fetopaties occur by the isolated congenital defects (pylorostenosis, megacolon,
megaloureter, agenesia, cystosis) and systemic congenital defects of the bone and
muscular tissues, skin.
Diabetic fetopathy
Diabetic fetopathy is the disease of the fetus due to maternal prediabetes and diabetes.
As a rule, the body mass is 4 - 6 kg.
The skin is purple cyanotic with small point hemorrhages, the neck is short, and the face
as well as the soft tissues of the back and chest are swollen.
The signs of immaturity are observed in the mature fetus.
Hepatomegaly and cardiomegaly can be seen.
160
Microscopic examination demonstrates hypertrophy of islets of Langerhars, increased
amount of B-cells, fat degeneration of the liver, glycogen accumulation in the tubular
epithelium in the kidneys, hydropic degeneration of the myocardium.
During the delivery of the child with fetopathy, hypoxia due to placenta vascular sclerosis
and disturbance of placental circulation may occur.
Hyaline membrane disease may develop because synthesis of surfactant is disturbed due
to disturbed lipid exchange (lipoproteid).
The death is caused by antenatal and intranatal asphyxia, respiratory insufficiency, birth
injury, and hypoglycemia after birth stress.
Cystic fibrosis (Fetal mucoviscidosis)
A disorder of exocrine glands, affecting both mucus-secreting and eccrine sweat glands
throughout the body, leading to viscid mucinous secretions and obstructive disease in
lungs, pancreas, liver.
A simple autosomal recessive syndrome; heterozygotes are unaffected.
The mutant gene may code for abnormal protein that affects chloride transport channels
across epithelial membranes.
General morphologic feature: obstruction by viscous mucoid secretions.
Pancreas: plugging and dilatation of ducts, atrophy of acini, and progressive fibrosis.
Liver: bile ducts plugged by mucus with biliary obstruction and fibrosis.
Lung: Obstruction and secondary infection of air passages, hyperplasia and hypertrophy
of mucus-secreting cells.
Salivary glands: progressive dilatation of ducts, squamous metaplasia, glandular
atrophy, and fibrosis.
Intestinum: obstruction by mucus (meconium ileus)
Endocardial Fibroelastosis
Primary myocardial metabolic/enzymic defect and congenital malformations take place.
It is characterized by focal to diffuse, cartilage-like fibroelastic thickening of the
myocardium, cardiomegaly due to hypertrophy of left ventricle.
Death can be due to acute cardiac insufficiency in first days of life or due to chronic
cardiac decompensation at connection (intercurrent) of the others diseases (pneumonia).
Alcogolic syndrome of fetus
Alcogolic syndrome of fetus occurs by signs of embryofetopathy.
Prematurity, small weight of fetus.
Tight forehead, flat bridge.
Narrow palpebral fissures.
Hyperthelorism, ptosis, epicanthus.
Cleft palate, short fingers.
Malformations of the heart, kidney, hip-joint.
Small cerebellum.
Decreased pulmonary surfactant.
Frequent mental retardation.
161
PERINATAL PATHOLOGY
Perinatal period means the period before and after the delivery, begining from the 22th
week of gestation (196th day) to the 1st week of extrauterine life.
Perinatal period and the respective pathology and mortality is divided into
1. Antenatal period (from the 22nd week of gestation to the delivery).
2. Intranatal period (during the delivery) begins in the moment of the delivery until the birth.
3. Postnatal (neonatal) period (from birth to 7th day after birth).
In the 22nd week of gestation the fetus must have the weight more than 500 g and the
length of the body – 25-30 cm. This fetus can live a life of independence.
Delivery of a smaller fetus and until the end of 22 week of the gestation is called abortion.
Stillborn is fetus without breathing and palpitation in the moment of the delivery.
Liveborn is infant with signs of the breathing and palpitation.
Perinatal mortality is stillborn and infant mortality within the first 7 days of the life.
Causes and morphological features of prematurity and overmaturity of newborns
Features of the prematurity
1. Premature infant is defined as those when the term of gestation is until 37 week.
2. Body weight is less then 500 gramms.
3. Body length is less then 25 cm.
4. Lanugo hair on the face.
5. Underdeveloped nails.
6. Soft lobe of the ear.
7. In girls the labia minora and clitoris are not covered by labia majora.
8. In boys the testes are not descended, scrotum isn’t wrinkled.
9. Soft cranial bones.
10. Beclard’s nucleus of the distal femoral epiphysis is absent (in mature children it is 5 - 7 mm).
Microscopic examination of the organs in premature fetus allows determining the degree
of prematurity:
1. Embryonic glomeruli are noted in the superficial layer of the cortical substance of the kidneys.
2. Erythroblastosis foci are observed in the kidneys and liver.
3. Thickening of interalveolar septa in the lungs.
4. Sprout zone in the brain is widened.
Main causes of the prematurity:
1. Diseases of the genital tract of the pregnant women.
2. Placental insufficiency.
3. Early agein of placenta.
4. Placental infections.
5. Acute and chronic infections of the pregnant women.
6. Severe toxicosis of pregnancy (nephropathy, eclampsia).
7. Rh antigens incompatibility.
Features of the overmaturity
1. Overmaturity infant is defined as termination from the 41st week of gestation and later.
2. Epidermis is dry, peeling, macerated, yellow or yellow-greenish color.
3. Very dense cranial bones.
4. Dense long lobe of the auricle.
5. Long nails.
6. General hypotrophy of fetus.
7. Decreased amount of amniotic fluid.
8. Amniotic fluid, umbilical cord, amniotic membranes are usually staining with meconium (yellowgreenish color) as the fetus experiences intrauterine hypoxia.
9. Beclard’s nucleus has 8 mm and more).
10. Signs of the placenta’s agening (placental infarction, petrification of the placenta).
Main cause of the overmaturity is late prime-gravide.
Overmaturity may lead to antenatal and intranatal death of the fetus due to hypoxia.
162
The most important diseases of perinatal period
Asphyxia (anoxia)
Asphyxia means lack of oxygen and excess of carbon dioxide in the blood suppling.
Asphyxia may be
1. Antenatal asphyxia is called the intrauterine asphyxia of the fetus (fetal asphyxia)
and arises during pregnancy.
2. Intranatal asphyxia (asphyxia neonatorum) arises during delivery.
3. Postnatal asphyxia (asphyxia neonatorum) is called asphyxia of the newborn and
arises after delivery in result of illnesses of newborn.
Causes of asphyxia
I. Causes of antenatal asphyxia associated with maternal factors resulting in decreased placental blood flow
and placental insufficiency, such as:
Maternal cardiovascular diseases.
Late toxemia of the pregnancy.
Chronic renal and pulmonary diseases of the mother.
Endocrine diseases (diabetes mellitus, thyreotoxicosis).
Accidental hemorrhage.
Severe infections of the mother.
Social causes (narcomania, alcohol, heavy cigarette smoking).
II. Causes of intranatal asphyxia associated with defects of the placenta and complications of the delivery:
Pretermed placental separation.
Placental abruption.
Placenta previa.
Prolaps of the umbilical cord.
Cord knots.
Multiple gestations.
Complications of the delivery: weakness of the delivery processes, fetus breech
presentation, disproportion between the fetus’ head and delivery tract of mother (narrow
pelvis, large fetus), rupture the short umbilical cord, umbilical cord winding round the
neck of fetus.
III. Causes of postnatal asphyxia associated with disturbance of the breathing.
As a rule, postnatal asphyxia is a continuation (or consequence) of intranatal asphyxia.
When during the delivery CNS (including respiratory center) is damaged or under the
influence of intrauterine hypoxia, the fetus makes the first inspiration intrauterinally
(carbon dioxide stimulates the respiratory center) and amniotic content is aspirated. In
this case the alveoli cannot spread after the delivery; postnatal asphyxia develops.
Brain trauma or edema with suppression of the respiratory center.
A birth injury of the spinal cord and central nervous system.
Infections of newborn.
Aspiration pneumonia.
Background: progressive fetal asphyxia.
Besides, fetal factors import for all types of asphyxia, because they lead to an inadequate
supply of nutrients from the mother, to inadequate of the delivery and may lead to birth injury.
Prominent among such fetal conditions are chromosomal disorders, congenital anomalies, and
congenital infections.
The main clinical-morphological syndromes of asphyxia
Hemorrhagic syndrome: petechial hemorrhage in brain, adrenal glands, serous
membranes and mucosa, congestion, dark fluid blood in the heart chambers, development
of syndrome’s disseminated intravascular coagulopathy on the other hand, resulting in
fibrin thrombi in the microcirculatory vesells.
Edematous syndrome in inner organs and cavities.
Degenerative changes in the liver, kidneys, myocardium, brain.
Aspiration of amniotic fluid containing skin epithelium, lanugo, meconium.
If such children survive, the disturbance of the psychomotor development and
cardiosclerosis may develop.
Pneumopathies
163
Pneumopathies are noninflammatory pulmonary diseases, which lead to asphyxia of
newborn. They occur as a rule in preterm children. There is atelectasis, development of the hyaline
membranes, edematous-hemorragic syndrome.
Atelectasis
Atelectasis in the newborn or primary atelectasis is defined as incomplete expansion of
a lung or part of a lung.
Stillborn infants have total atelectasis, while the newborn infants with weak respiratory
action develop incomplete expansion of the lungs and clinical atelectasis.
The common causes are prematurity, cerebral birth injury, CNS (central nervous system)
malformations and intrauterine hypoxia.
Macroscopicall appearance of atelectasis:
Large plural space.
Small and collapsed lungs against vertebral column and are of cyanotic color.
Slitlike air spaces.
Heart and great vessels fully exposed.
Microscopic examination demonstrates collapsed alveoli.
While pulmonary collapse or primary atelectasis is the term used for reduction in lung size
of a previously expanded and well-aerated lungs.
Secondary atelectasis in children and adults may occur from various causes such as
compression, obstruction, contraction and lack of pulmonary surfactant. Collapse may be
of the following 3 types:
1. Compressive collapse. Pressure from outside causes compressive collapse e.g. by
massive pleural effusion, hemothorax, pneumothorax, intrathoracic tumor, high
diaphragm and spinal deformities.
2. Obstructive/absorptive collapse. Obstruction of a bronchus or many bronchioles
causes absorption of oxygen in the affected alveoli followed by collapse e.g. by viscid
mucus secretions in bronchial asthma, chronic bronchitis, bronchiectasis, bronchial
tumors and aspiration of foreign bodies.
3. Contraction collapse. This type occurs due to localised fibrosis in lung causing
contraction followed by collapse.
Edematous hemorrhagic syndrome
It is associated with asphyxia when the lung capillaries are overfilled with the blood,
vascular permeability increases due to hypoxia.
Diffuse edema and large intra- and extraalveolar hemorrhages develop.
Difficulty of the breathing takes place; the children die because of respiratory
insufficiency.
The disease of hyalin membranes often accompanies this condition.
Autopsy demonstrates large lungs with hemorrhages.
Microscopic examination shows intraalveolar pink fluid, hemorrhages.
Respiratory distress syndrome of newborn (RDS)
RDS is one of the most common life threatening complications to confront the newborn
infant.
RDS is also known as hyaline membrane disease (HMD), highlighting one of the major
pulmonary anatomic findings in this disease.
Lungs are involved, asphyxia develops quickly, and the newborns die within the period of
24 - 36 hours.
It can have many origins, including:
1. Excessive sedation of the mother with consequent depression of respiration in the
infant;
2. Brain injury with failure of the central respiratory centers;
3. Feeble respiratory efforts secondary to immaturity of the lungs and sceletal muscles
(primary atelectasis);
4. Aspiration during birth of blood clot and amniotic fluid when the amniotic debris
(i.e., desquamated keratotic squames, mucus, lanugo hairs, proteinaceous precipitate,
164
and blood) blocks ventilatory function;
5. Asphyxiating coils of umbilical cord about neck of the infant. But more important
than all these by an order of magnitude is the idiopathic RDS.
The fundamental defect in RDS is a deficiency of pulmonary surfactant. Surfactant reduces
surface tension within the alveoli so that less pressure is required to hold alveoli open, and it
maintains alveolar expansion by varying surface tension with alveolar size. It is synthesized by type 2
alveolar cells most abundantly after the 35 week of gestation in the fetus. At birth, the first breath of
life requires high inspiratory pressures to expand the lung. With deficiency of surfactant the lungs
collapse with each successive breath as it did with the first.
Surfactant synthesis is modulated by a variety of hormones, including cortisol, insulin,
prolactin, and thyroxin. The role of glucocorticoids is particularly important. Corticosteroids induce
the formation of surfactant lipids and apoproteins in fetal lung. Surfactant synthesis may be
suppressed by the infants of diabetic mothers’ compensatory high blood levels of insulin, which
counteracts the effects of steroids. This may explain why infants of diabetic mothers have a higher risk
of developing RDS.
Morphological features of RDS
Gross examination of the lungs. Although of normal size, they are solid, airless, reddish
purple like the liver, and they usually sink in water. Autopsy demonstrates stiff, congested and heavy
lungs.
Microscopic examination:
The alveoli are poorly developed, and those that are present are collapsed. The atelectasis
results from the clearance of the fluid without its replacement by air.
Interstitial and intraalveolar edema.
In early stage of RDS the necrotic cellular debris is present in the terminal bronchioles
and alveolar ducts.
Later, the necrotic material becomes incorporated within pink hyaline membranes that
line the respiratory bronchioles, alveolar ducts, and random alveoli, mostly the proximal
alveoli.
The membranes are largely made up of fibrinogen and fibrin admixed with cell debris
derived chiefly from necrotic alveolar-lining pneumocytes.
In infants who survive more than 48 hours reparative changes are seen in the lungs. The
alveolar epithelium proliferates under the surface of the membrane, which may be
desquamated into the airspace, where it may undergo partial digestion or phagocytosis
by macrophages.
Infants who recover RDS are at increased risk for developing a variety of other complications
stiff, as well. Most important among these are patent ductus arteriosus, intraventricular hemorrhage,
and necrotizing enterocolitis. Thus, although the high technology of today saves many infants with
RDS, it also brings to the surface the exquisite fragility of the immature neonate.
Pneumonia in newborn
Pneumonia of newborns may occur in uterus (in ante- and intranatal periods) as well as
after the birth. The etiology is different. The most frequent are coccal pneumonias,
klebsiella, and colon bacillus. The disease often develops against the background of
amniotic fluid aspiration both infected and not infected.
The most often in newborn the aspiration pneumonia develops.
Aspiration syndrome is the first inspiration done in uterus. Amniotic fluid may be infected
or may contain meconium.
The syndrome is due to hypoxia, and is often observed at overmaturation.
If the child survives for 3 - 5 hours, small-focal pneumonia develops, in 24 hours it turns
into confluent pneumonia.
In massive aspiration, total or disseminated atelectasis of the lungs (primary) may
develop as the lungs are filled with aspiration masses and do not spread.
Microscopically, leukocytic and monocytic infiltration of alveolar tissue involving the
bronchioles and bronchi are observed. Elements of amniotic fluid are determined in the
exudate.
It is considered that intrauterine pneumonia is responsible for the death during the first 1
-3 days of life.
Birth injury
165
Birth injuries constitute important causes of illness or death in infants as well as in
children during the first years of life.
Morbidity associated with birth injury may be acute or the result of later-appearing
sequels.
Birth injuries are damage to the fetal tissues and organs with mechanical forces during
the delivery. Birth injury should be differentiated from obstetric injury, which occurs
when obstetric manipulations are carried out.
Causes of birth injury are due to:
1. The state of the fetus:
a) Embryopathy.
b) Fetopathy.
c) Prematurity (the tissues are easily ruptured) and overmaturity (hypoxia increased
vulnerability of the fetus).
2. The state of the maternal passages:
a) Rigidity of the birth canal tissue.
b) Pelvis defects (narrow pelvis, rachitic pelvis, anomalies, tumors, wounds)
c) Tumors of maternal passages
d) Oligoamnios, hydramnion.
3. The state of failure of the delivery’s dynamic:
a) Precipitated delivery.
b) Prolonged delivery.
c) A lot or little of amniotic fluid.
Pathology
Cephalogematoma is produced by an effusion of blood between the pericranium and one
of the bones of the head.
It disappears slowly. When infected, it may become a source of purulent meningitis. The
most severe intracranial injury is hemorrhage to the meninges and brain substance.
All hemorrhages are divided into
1. Epidural hemorrhages are located between the bones of the skull and the dura mater
(inner cephalohematoma).
2. Subdural cephalohematomas occur in rupture of the falciform process and
cerebellum tentorium. The blood is accumulated under the dura mater on the brain
substance.
3. Subarachnoid cephalohematoma is localized between the arachnoid and pia mater. It
occurs in rupture of the falciform process, cerebellum tentorium, veins.
4. Intracerebral cephalohematomas are the hemorrhages to the vascular plexi of the
brain, under the ependyma of the lateral ventricles with rupture to the ventricles.
The most frequent course of the death in intracranial injury is rupture of the falciform
process and cerebellum tentorium.
Caput succedaneum and cephalhematoma are also so common, even in normal
uncomplicated births, that they hardly merit the designation “birth injury”. The first refers
to progressive accumulation of the interstitial fluid in the soft tissues of the scalp, giving
rise to a usually circular area of edema, congestion, and swelling at the site where the
head being to enter the lower uterine canal. Because the fluid accumulates in the
subcutaneous tissue, it may extend across the suture lines.
Hematoma of the sternomastoid muscle may follow traction on the head during the birth
of the shoulders, or the extraction of the after-coming head.
Visceral hemorrhages: subcapsular hematomas and hemorrhage from spleen, liver,
stomach due to fracture.
The most often fractures of the spine (often fatal to the life), fracture of the clavicle, bone’s
skull, femoral and humerus bones occurring in large fetuses.
Hemolytic disease of the newborn (HDN) or Erythroblastosis fetalis
Erythroblastosis fetalis is defined as a hemolytic disease in the newborn caused by
ABO-group and Rh incompatibility between mother and child.
When the fetus inherits red cells antigenic determinations from the father that are foreign
to the mother, a maternal immune reaction may occur, leading to the hemolytic disease in
166
the infant. Basis to such a phenomenon are leakage of fetal red cells into the maternal
circulation and, in turn, transplacental passage of the maternal antibodies into the fetus.
Any of the numerous red cell antigenic systems may theoretically be involved, but the
major antigens known to induce clinically significant immunologic disease are the ABO
and certain of the Rh antigens (Rh negative mother and Rh positive fetus)
The resultant fetal hemolytic reaction may cause mild-to-severe disease in the newborn,
or even death.
A hemoliyic disease that may appear during gestation or shortly after delivery.
Classification of erythroblastosis fetalis
1. Congenital hydrops (edematous form) is characterised by edema of skin, subcutaneous fat,
meninges and brain substance, there is transudation in the cavities. Microscopically: erythroblastosis
in the liver, spleen, lymphatic nodes, and kidneys. Signs of immaturity of organs in mature newborns
can be found.
2. Anemia neonatorum (hemolytic anemia) is frequent in immature fetuses. The skin and mucous
membranes are pale. Jaundice is absent. Hepatosplenomegaly take place. In the mildest form, the
anemia may be only slight, and the child may survive without further complications. More severe
hemolysis dives rise to jaundice and other features associated with hemolytic anemias.
3. Icterus gravis (severe jaundice of the newborn) is evident by the end of the first day. The disease
develops quickly. The most serious threat in this disease is central nervous system damage known as
kernicterus. In jaundiced infants, the unconjugated bilirubin appears to be particularly toxic to the
brain tissue. The brain is enlarged and edematous and, when sectioned, is found to have a bright
yellow pigmentation (kernicterus). The cells stain by yellow color. The liver and spleen are enlarged;
they have the signs of erythroblastosis and hemosiderosis. There are bilirubin infarcts in the kidneys.
Histologically in all forms, the diagnosis of erythroblastosis depends on the identification
of abnormally increased erythropoetic activity in the infant. The red cells series in the
marrow is hyperactive and extramedullary hematopoesis is almost invariably present in
the liver, spleen, and possibly other tissues, such as lymph nodes, kidneys, lungs, and even
in the heart.
In early massive immunization of the mother, early fetopathy develops. The fetus dies
before the birth, on the 5th - 7th month of gestation.
When the mother’s immunization is later and more moderate, the child is born alive with
one of the forms of HDN.
In children who survived HDN, defects of CNS development (including complete
idiopathy) may occur in future.
Sudden infant death syndrome (SIDS)
Included here since some cases of SIDS may be related to congenital cardiac disorders.
Defined as sudden and unexpected death in a previously basically well infant, when the
cause of death cannot be explained even after autopsy.
Probably a multifactorial entity or common end point of diverse derangements.
Ninety per cent of SIDS deaths occur in the first 6 months of life, most between the ages of
2 and 4 months. The deaths occur without a struggle during the night after a period of
sleep.
Often there are minor antecedent respiratory tract infections.
Causes of death are unknown. Among innumerable hypotheses, those favored are:
- Cardiac arrhythmias.
- Disturbed regulation of respiration, “the apnea hypothesis”.
- Inherited disorders of fat oxidation.
- Unsuspected intestinal infection with Clostrium botulinum.
- Defective regulation of body temperature with resultant acute malignant
hyperthermia.
Morphology
A variety of changes of uncertain significance.
Abnormalities in the myocardial conduction system have been observed, not always
present and of diverse nature.
Subtle medial thickening of small pulmonary arteries and brain-stem gliosis suggests
chronic hypoxia.
167
Right ventricular hypertrophy may be secondary to pulmonary vascular changes or a
primary anomaly.
Also seen are retention of fetal hemoglobin, extramedullary.
168
INFECTIOUS DISEASES
Infectious diseases are those caused by infectious agents (viruses, bacteria, fungi). Protozoa
and helminths cause invasive diseases. Infectious diseases have a number of common features.
Clinical-morphological characteristics of infectious diseases
Each infectious disease has its own causative agent, which can be isolated from the blood
or excreted materials of the patient.
In infectious, one can observe formation of a primary infectious complex consisting of a
primary affect, lymphangitis and lymphadenitis.
The route of the infection from the primary focus or complex may be lymphogenic,
hematogenic, intracanalicular, and perineural, contact.
Each infectious disease is characterized by local changes in the portal of entry of infectious
agent.
A number of common changes (rash, vasculitis, hyperplastic processes in the lymphatic
nodes, spleen, bone marrow, inflammatory processes in the interstitial tissue and
degenerative changes in the parenchymatous organs) are observed in infectious diseases.
Infectious diseases are chiefly cyclic. The course of the infectious disease is divided into
incubative and prodromal periods and the period of main manifestations of the disease
(phases of increase of the signs, climax and extinction).
Infections can be either exogenic or endogenic.
Infectious diseases are classified according to a number of signs.
Biological classification:
Anthroponoses - infectious diseases typical only for people.
Antropozoonoses - infectious diseases that can develop both in people and animals.
Biocenoses - a group of anthroponoses and anthropozoonoses transmitted through the
bites of insects, which become the place of causative agent multiplying.
According to the etiology they are divided into
Viral.
Rickettsiosis.
Bacterial.
Fungal.
Protozoal.
Parasitogenic.
Classification according to the mechanism of transmission:
Intestinal.
Respiratory.
Blood (transmissive) transmitted by blood-sucking insects.
Infections of the external integument, {subcutaneous fat and muscles).
Infections with different mechanisms of transmission.
According to the duration there are
Acute.
Chronic.
Latent.
VIRAL DISEASES
Respiratory disorders are caused by a wide variety of viruses, of different families, species,
and serotypes. These include:
1. Orthomyxoviruses (influenza A, B, and C – RNA virus).
2. Paramyxoviruses (respiratory syncytial virus, parainfluenza viruses, measles virus, mumps
virus)
3. Adenoviruses.
4. Herpesviruses (varicella-zoster virus).
169
5.
6.
7.
8.
Cytomegalovirus.
Herpes simplex virus (HSV).
Picornaviruses (rhinoviruses, echoviruses, and coxsackieviruses).
Human respiratory coronaviruses.
The peculiarities of viral diseases
1. Being highly contagious, viruses cause epidemics and pandemic.
2. The variety of viruses determines the lesion of specific cells due to the virus trophism. The
character of the cell receptors determines trophism.
3. The duration of the disease depends both on the type of the virus and the reactivity of the
macroorganism and can be acute, chronic, slow.
4. The morphological manifestations of cell-virus interrelations are:
a) Cytolytic effect of the virus on the cell (influenza).
b) Formation of inclusions in the cell (influenza, adenovirus infection).
c) Integration of the virus and the cell genome without considerable destruction of the
cell (hepatitis B, HIV infection).
d) Proliferation of target cells (smallpox).
e) Giant-cell transformations (measles).
Acute respiratory viral infection (ARVI)
The term denotes a group of acute inflammatory diseases caused by pneumotropic viruses
(Influenza, parainfluenza, adenovirus infection, respiratory sincitial virus, rhinovirus, reovirus).
Influenza
Influenza is a disease characterized by abrupt onset, fever, sore throat, headache, muscle
pains, and acute toxic state. Dry cough and nasal discharge are present but usually are
overshadowed by systemic symptoms. In uncomplicated cases the illness lasts a few days,
but pulmonary complications such as influenza pneumonia and secondary bacterial
infection of the lung may complicate and prolong course.
Influenza viruses are highly contagious and afflict people of all ages and have 3 types:
a) Influenza A virus, the most common cause of viral pneumonia in adults, infects
animals and man and produces pandemics.
b) Influenza B virus is apparently restricted to man, causes epidemics, and is associated
with Reye’s syndrome in children and pneumonitis and croup in infants.
c) Influenza C virus causes sporadic upper respiratory infections, but not epidemic
influenza. Influenza has significant mortality and morbidity, and may have long-term
sequelae.
Patients with viral influenza during the third trimester of pregnancy, the aged, and
persons with valvular heart disease or chronic bronchopulmonary disease all have
increased susceptibility to bacterial superinfection. Superinfection usually occurs 1 to 5
days after the onset of the viral illness, while the patient appears to be getting well.
At present differential diagnosis of ARVI is not difficult. Immunomorphological study
with antisera to the definite strain of viruses is performed in the smears from the mucous
membrane of the upper respiratory tract or in the tissue (if it is autopsy material). In this
case bright fluorescence is seen under the microscope.
Influenza viruses are transmitted by aerosols generated by coughing and sneezing.
The incubation period is 2 - 4 days.
The virus invades the bronchial and alveolar epithelium and endotheliocytes of the
capillaries and multiplies there causing primary viremia. The epithelial cells die, the virus
leaves them and invades ones more the bronchial and alveolar epithelium. At this stage
acute bronchitis or tracheitis develops. These are the first clinical signs of the disease.
The development of the virus in the cells causes degeneration, necrosis and desquamation
of the bronchial epithelium, which in turn causes secondary viremia.
Its manifestations are vasoparalytic action (plethora, stasis, hemorrhage) and immunedepressive action (phagocytosis inhibition, chemotaxis, etc.), which contributes secondary
(often bacterial) infection.
Morphology
Clinical-morphological forms of influenza
170
1. Slight influenza is characterized by the lesion in mucosa of the upper respiratory tract (edema,
hyperemia, serous inflammation). Laringitis, tracheitis and bronchitis occur. These are microcolonies
of the virus; they also can be determined with immunomorphological method. The duration of the
disease is 5 - 6 days with following convalescence.
2. Mild influenza is characterized by involvement of the pathological processes in mucosa of the
bronchi, bronchioles and lungs.
The histopathologic features include a necrotizing tracheitis and bronchitis; diffuse
hemorrhagic necrotizing pneumonitis with pulmonary edema.
Ciliated epithelial cells are destroyed and goblet cells and mucous glands disrupted.
Individual cells show pycnosis of the nuclei and loss of cilia.
Interstitial pneumonia develops. Interalveolar septa are thickened due to proliferation
with lymphocytes and macrophages.
Bronchioles become thickened, distended, and infiltrated with mononuclear cells. There is
often severe inflammatory edema, and a fluid exudate in the alveolar spaces has a hyaline
membrane appearance.
Desquamation of the alveolocytes lead to decrease of surfactant and development
surfactant-depending atelectases in the lungs.
The duration of the disease is 3 -4 weeks.
3. Severe influenza is characterized by the complicated duration of disease and occurs by:
1. Severe toxicosis.
Besides serous hemorrhagic bronchitis and pneumonia, hemorrhagic lung edema may
develop. Hemorrhage to the brain and internal organs develop.
The patients die on, the 4th - 5th day of hemorrhage to vital centres and of acute
respiratory and cardiovascular insufficiency.
2. Pulmonary complications.
If bacterial superinfection occurs, the picture is indistinguishable from that of ordinary
bronchopneumonia or lobar pneumonia.
Lungs are dark red and firm with interstitial emphysema (pink color) that may extend
into the mediastinal tissue. Necrotic foci, abscesses are presence also. Lungs appear as
(“large variegated (motley) influenza lung”).
Microscopically, there is pronounced sloughing of the bronchial epithelium into the
bronchial lumens.
Encephalitis, serous meningitis, brain edema, trunk dislocation develop in the brain.
Obliterating bronchitis, bronchiolitis, bronchiectasis, pneumofibrosis and other chronic
lung diseases develop.
Degeneration and inflammation in the nodes of the vagus and sympathetic nerves cause
neuritis.
Myocarditis and pericarditis, as well as encephalitis, might be found as postmorten
examination of fatal cases, but they are uncommon.
The death is caused by intoxication, cerebral hemorrhages, brain edema, brain trunk
dislocation, pulmonary complications (pneumothorax, empyema), and cardiovascular
and pulmonary insufficiency.
Paramyxoviruses
Paramyxoviruses (parainfluenza viruses, respiratory syncytial virus, measles virus,
mumps virus) are important causes of respiratory disease in infants and young children.
Paramyxoviruses are spherical enveloped viruses that contain single-stranded RNA.
They are transmitted by inhalation of droplets of aerosols.
Parainfluenza viruses infection (Types l-4)
Type 3 parainfluenza is the most prevalent of the parainfluenzas, occurring endemically
throughout the year.
Infants are especially susceptible.
Parainfluenza viruses are spread principally by direct contact or by large droplets (in
contrast to the spread of influenza virus by inhalation of small droplets).
Replication is restricted to the respiratory tract and moderate intoxication.
The infection involves only the upper respiratory tract, except in some infants in whom the
primary infection may also involve the larynx, trachea, and bronchioles.
The pathology of the disease resembles slight influenza.
171
The characteristic signs are tracheal and bronchial epithelium proliferation, appearance
of polymorphic cells with one or several picnotic nuclei (multinucleated cells).
Edema in larynx may lead to development of the “false croup” and asphyxia.
Complications are secondary infection, bronchopneumonia, asphyxia, angina, sinusitis,
and otitis.
Respiratory Syncytial Virus infection
The respiratory syncytial virus is the most common cause of viral pneumonia in children
under 2 years of age and is a common cause of death in infants aged 1 to 6 months.
This agent accounts for about one-third of hospital admissions for pneumonia and for up
to 90% of those admitted for bronchiolitis.
Susceptibility is also increased in elderly or immunocompromised patients.
In temperate climates of the northern hemisphere, annual epidemics occur in midwinter
(January-March).
Histopathologic features include necrotizing bronchitis, bronchiolitis, and interstitial
pneumonia.
The infiltrate is purely mononuclear (predominantly lymphocytes). In many cases
irregular intracytoplasmic inclusion bodies are seen in alveolar and bronchiolar epithelial
cells, but intranuclear inclusions are not present.
The death is caused by asphyxia and pulmonary complications.
Adenovirus infection
Adenovirus infection (AVI) is caused by DNA-containing adenovirus. This is characterized
by invasion of the upper respiratory tract, lymphoid tissue of the intestine, abdominal
lymphatic nodes as well as conjunctivitis.
Adenoviruses (subgroup B, types 4 and 7) are common causes of acute respiratory disease
and adenovirus pneumonia in military recruits coming together for the first time for basic
training.
Adenoviruses (subgroup C) are also important causes of chronic pulmonary disease in
infants and young children.
The course may be slight and severe.
1. Slight AVI is characterized by acute rhinitis, laryngitis, tracheobronchitis, acute
pharyngitis, conjunctivitis and regional lymphadenitis. Histopathologic features of adenovirus
pneumonitis include necrotizing bronchitis and bronchiolitis, with necrosis and desquamation of the
epithelium Sloughed epithelial cells are subsequently mixed with mononuclear cells, mucus, and cell
debris, so that the damaged bronchiole resembles a thrombosed blood vessel. There is interstitial
pneumonia, with areas of consolidation showing extensive necrosis, hemorrhage, edema, and
mononuclear inflammatory infiltrate. Two distinctive types of intranuclear inclusions - smudge cells
and Cowdry type A intranuclear inclusions - are scattered throughout the lesions but primarily involve
bronchiolar epithelial cells and alveolar lining cells.
2. Severe AVI is caused by generalization of the virus and secondary infections. In
generalized infection, the viruses multiply in the epithelium of the intestine (diarrhea), kidneys, liver,
pancreas, ganglious cells of the brain with development of inflammation and hemorrhages. Secondary
infection is characterized by suppuration and sepsis.
Complications: otitis, sinusitis, tonsillitis, pneumonia due to the secondary infection. The
cause of death is pneumonia, sepsis.
Measles
Measles is an acute highly contagious infectious disease characterized by catarrhal
inflammation of the mucous membranes of the upper respiratory tract, conjunctiva and spotted
papular eruption on the skin.
Etiology and pathogenesis
The causative agent of measles is an RNA-containing virus transmitted by inhalation (airdroplets).
The virus enters the upper respiratory tract and eye conjunctiva.
Degenerative changes in the epithelium of the mucous membrane and hematogenous
spread are accompanied by short viremia resulting in dissemination of the virus in the
lymphoid tissue, which in turn causes immune reconstruction.
172
Viremia becomes more expressed and prolonged, the eruption appears. When the eruption
disappears, the virus cannot be found in the organism.
Incubation period is 9-11 days. The duration of the disease is 2 - 3 weeks.
The disease produces stable immunity.
Morphology
Catarrhal inflammation in the mucous membrane of the mouth, trachea, bronchi,
conjunctiva develops.
The mucous membrane is swollen, plethoric; the mucous secretion is increased, which is
accompanied by rhinitis, cough, and lacrimation.
Severe cases are accompanied by necroses; the mucosa becomes dull, greyish-yellow;
small lumps are seen on its surface.
The edema and necrosis of the laryngeal mucosa can develop reflex spasm of its muscles
with asphyxia (so called “false croup”).
Measles is characterized by metaplasia of mucosa epithelium into multilayer squamous
epithelium observed in early periods (5th - 6th days of the disease), which decreases the
barrier function of the epithelium.
Varies from pure interstitial (viral) pneumonia to lobar (bacterial) pneumonia. There are
often pathognomonic multinucleated giant cells (Warthin-Finkeldey cells), intranuclear
and intracytoplasmic inclusions, and hyperplasia of distal bronchial cells. In
immunocompromised patients measles pneumonia may occur without rash and is often
fatal.
Viremia and generalization of the process result in enanthema and exanthema:
1. Enanthema is noted on the mucous membrane of the cheeks against the lesser lower
molars. It looks like whitish spots called Belsky-Filatov-Koplik spots, which develop
before the eruption on the skin. They are of great diagnostic significance.
2. Exanthema in the form of large-spot papular eruption first appears on the skin
behind the ears, then on the face, neck, body, and inner surface of the extremities.
Complications is accompanied with the secondary viral and bacterial infection.
Destructive (necrotic or purulent-necrotic) panbronchitis can occur.
The disease involves internal membrane of the bronchi (endobronchitis), middle layer
(mesobronchitis), and external layer (peribronchitis).
On incision, the involved lungs look like grey-yellow foci resembling tuberculosis ones.
Such panbronchitis is the source of bronchiectasis, lung abscess, and purulent pleurisy.
The involvement of peribronchial lung parenchyma causes the development of
peribronchial pneumonia and chronic disease of the lungs resulting in pneumosclerosis.
Moist gangrene of the soft tissue of the face (noma) is rarely observed at present.
The death of the patients with measles is associated with pulmonary complications and
asphyxia in “false croup”. Modern seroprophylaxis and vaccination have resulted in considerable
reduction of the frequency of disease and death rate.
Mumps
Mumps virus causes a transient inflammation of the parotid glands and rarely of the
testes, pancreas and central nervous system. Mumps viruses are spread by inhalation
(air-droplets) and multiply within respiratory epithelial cells, salivary glands, and T cells in lymph nodes.
A transient viremia spreads the mumps virus to other glands and the central nervous
system via the choroid plexus.
Morphology
In mumps (parotitis), which is bilateral in 70% of cases, affected glands are enlarged,
have a soft consistency, and are moist, glistening, and reddish brown on cut-section.
Microscopically, the gland interstitium is edematous and diffusely infiltrated by
histiocytes, lymphocytes, and plasmocytes that compress acini and ducts. Neutrophils and
necrotic debris may fill the ductal lumen and cause focal damage to the ductal epithelium.
In mumps orchitis, testicular edema, mononuclear cell infiltration, and focal hemorrhages
has been revealed.
Complications: atrophy of testis with azoospermia development, serous meningitis and
meningoencephalitis.
173
The death of the patients with mumps is associated with central nervous system
involvement.
ACQUIRED IMMUNE DEFICIENCY SYNDROME (AIDS)
Etiology and pathogenesis
Human immunodeficiency virus (HIV) is the causative agent for AIDS.
HIV is a retrovirus that contains only RNA. HIV is a sexually transmitted disease.
Infection is aided by Langerhans cells in mucosal epithelial surfaces, which can become
infected.
Infection is also aided by the presence of other sexually transmitted diseases that can
produce mucosal ulceration and inflammation.
The CD4+ T-lymphocytes have surface receptors to which HIV can attach to promote
entry into the cell. The infection extends to lymphoid tissues which contain follicular
dendritic cells that can become infected and provide a reservoir for continuing infection of
CD4+ T-lymphocytes.
HIV can also be spread via blood or blood products, most commonly with shared
contaminated needles used by persons engaging in intravenous drug use.
Mothers who are HIV infected can pass the virus on to their fetuses in utero or to infants
via breast milk.
The source of HIV is a sick person or a virus carrier. The patients are infective during all
the life.
When HIV infects a cell, it must use its reverse transcriptase enzyme to transcribe its RNA
to host cell proviral DNA. It is this proviral DNA that directs the cell to produce additional
HIV virions, which are released.
When the CD-4 lymphocyte count drops below 200/microliter, then the stage of clinical
AIDS has been reached. This is the point at which the characteristic opportunistic
infections and neoplasms of AIDS appear.
Clinical picture
Incubative period of HIV-1 at sexual way of infection lasts from 2-3 weeks to 2-3 months,
sometimes I year.
The early signs of the disease are increase of temperature, cough, nausea, vomiting,
diarrhea, presence of antibodies to HIV infection with simultaneous loss of body mass (20
kg during the last 2-3 years).
After those lymphatic nodes of different localization as well as the liver and spleen
enlarge, lymphopenia and hypergammaglobulinemia develop. The signs of the disease
also depend on the lesion in the definite system, i.e. meningoencephalitis, pneumonia,
gastritis, duodenitis, nephritis.
Next stage is appearance of oncological diseases or generalized infection.
The clinical spectrum of HIV infection is now recognized to comprise
1. Acute viral infection sometimes associated with immune complex disease.
2. Persistent generalized lymphadenopathy.
3. Chronic active viral infection with constitutional symptoms or AIDS related
complex.
4. Immunodeficiency leading to opportunistic infections or tumors (AIDS).
5. Chronic encephalopathy caused by HIV
6.Chronic active viral infection with immunocomplex disease (such as
thrombocytopenic purpura).
The signs of suspected AIDS (according to WHO)
Prolonged fever of unclear origin.
Chronic diarrhea (not less than 2 months).
Unexplainable body weight loss (by 10% or more).
Pneumonia of unclear origin resistant to standard therapy.
Lymphopenia.
There are AIDS-ihdicating diseases (according to WHO)
Candidosis of the esophagus, trachea, bronchi, lungs.
Extrauterine cryptococcosis.
174
Cryptosporiosis with diarrhea for more than 1 month.
Pneumocyst pneumonia.
Cytomegalovirus lesion of some organs (except for the liver, spleen, lymphatic nodes in
the patients over 1 month).
Infection caused by herpes simplex, persisting more than I month in the patients aged
over 1 month.
Toxoplasmosis of the CNS in the patients aged over 1 month.
Malignant lymphomas seen with AIDS are typically of a high grade and extranodal, often
in the brain. They are very aggressive and respond poorly to therapy.
Kaposi’s sarcoma (KS) produces reddish purple patches, plaques, or nodules over the skin
and can be diagnosed with skin biopsy. Visceral organ involvement eventually occurs in
3/4 of patients with KS.
Morphology
At autopsy the gross pathology of AIDS can be split into three general categories as follows:
1. The morphologic manifestations of profound lymphoid depletion.
2. Infections caused by opportunistic pathogens.
3. Unusual neoplasms such as Kaposi’s sarcoma and high-grade lymphoma.
The early stage of HIV is characterized by enlarged lymph nodes and the follicular
hyperplasia.
With disease progression, the frenzy of B-cell proliferation subsides and gives way to a
pattern of severe follicular involution. The follicles are depleted of cells; and the organized
network of follicular dendritic cells is disrupted. The germinal centres may even become
hyalinised. These “burnt-out” lymph nodes are atrophic and small and may harbor
numerous opportunistic pathogens. In the empty-looking lymph nodes and in other
organs, the presence of infectious agents may not be readily apparent without the
application of special stains.
In later stages of AIDS, spleen and thymus also appear to be “wastelands”.
Non-Hodgkin’s lymphomas, involving the nodes as well as extranodular sites, such as the
liver, gastrointestinal tract, and bone marrow, are primarily high-grade diffuse B-cell
neoplasms.
Neurologic complications, especially the AIDS-dementia is an important cause of
morbidity in patients in advanced stages of infection. The pathologic abnormalities in
patients with AIDS-dementia complex are variable. Multinucleated cells in the brain are
found in a subgroup of patients with severe disease. These cells are derived from
macrophages and support viral replication. These are thus markers of productive
infection. All histopathlogic abnormalities are most prominent in the subcortical
structures, and besides multinucleated cells they include diffuse pallor of the white matter
and vacuolar myelopathy.
Lymphocytic meningitis is seen in patients around the time of seroconversion and is
defined as occurring in the absence of any demonstrable opportunistic pathogens.
HIV encephalitis is a multifocal process characterized by inflammatory foci including
multinucleated giant cells, mainly seen in white matter, basal ganglia and brain stem.
Diffuse poliodystrophy is the term applied to neuronal loss, microglial activation and
gliosis in CNS grey matter.
Cerebral vasculitis is seen most prominently in childhood HIV disease of the brain.
175
BACTERIAL INFECTIONS OF CHILDHOOD
Diphtheria (D)
Diphtheria is an acute infectious disease characterized by fibrinous inflammation in the
focus of primary fixation of the causative agent and general intoxication due to exotoxin absorption.
Etiology and pathogenesis
Diphtheria is caused by a slender, gram-positive rod Corynebacterium diphtheria, which
is passed from person to person via aerosols or traumatic skin.
Diphtheria is amenable to virtually complete eradication by routine immunization with
diphtheria toxoid. Even in medically advanced countries, however, diphtheria may occur
when immunization procedures break down because of war, complacency, or cultism.
Transmission to nonimmune individuals usually occurs by the respiratory route.
Diphtheria is a composite of a local inflammation and a systemic intoxication. Toxic
produced locally by toxigenic strains of C. diphtheriae is responsible for an inflammatory
reaction on body surfaces at the site of infection (usually the oral pharynx, from which the
process often extends to the nose or larynx).
Occasionally the tracheal, esophageal, or gastric mucosa is involved as well.
Less commonly, but particularly in the tropics, cutaneous trauma or burns may be the site
of diphtheria.
The umbilical cord (in diphtheria neonatorum), the genital tract, and the conjunctivae are
rare sites.
Incubation period is 2-10 days.
The diphtheria bacillus multiplies at the site of attachment on the mucosa and excretes
exotoxin. The exotoxin causes local necrosis of the epithelium, paretic dilatation of the
vessels with disturbance of their permeability, edema of the tissues and release of
fibrinogen from the vascular bed. Fibrinous films are formed on the surface of the
damaged mucous membrane.
Exotoxin affects cardiovascular, nervous systems and adrenal glands. This simultaneous
damage causes hemodynamic disturbances in the organism; excretion of the exotoxin
from the organism is accompanied by the damage of tubular epithelium of the kidneys.
The disease is more common in children; at present the disease is more frequent in
children over 7 years.
Morphology
Clinical-morphological classification
1. Diphtheria in the pharynx.
2. Diphtheria in the respiratory tract.
3. Rarely forms of diphtheria.
Diphtheria in the pharynx
Local changes:
Cervical adenopathy seems out of proportion to the pharyngeal lesion.
Soon small gray or white patches of exudate appear on the pharyngeal mucosa, usually
over the tonsils. These enlarge and coalesce and, with the accumulation of blood, become
gray or black. This exudate constitutes the characteristic diphtheritic inflammation, which
consists of leukocytes and numerous bacteria enmeshed in a dense network of fibrin.
The lymphoid tissues both in regional lymph nodes and systemically (as in the spleen)
undergo hyperplasia with the development of prominent germinal centers that are often
centrally necrotic.
The soft tissue of the neck is swollen. In severe toxic forms, the edema is considerable and
can involve the anterior surface of the chest.
General changes are accompanied with toxinemia and appear:
1. Diphtheria toxin is particularly toxic to myocardium.
Toxic myocarditis develops in the heart. Alterative and interstitial forms of myocarditis
are distinguished. The cavities of the heart are dilated; the muscle is dull, flabby,
variegated. Parietal thrombi can be observed.
176
In the early stages, interstitial edema, cloudy swelling of myocardial fibers, and the
accumulation of fine cytoplasmic granules of lipid are seen microscopically. The changes
of cardiomyocytes are characterized by fat degeneration and small foci of myolysis.
If myocarditis develops at the beginning of the 2nd week of the disease and the death is
caused by acute cardiac failure and an arrhythmia, the condition is called early
cardioplegia. Due to diphtheritic myocarditis the cardiosclerosis and congestive heart
failure develop.
2. Diphtherial toxin has a special affinity for peripheral nerves (often in glossopharyngeal nerve,
diaphragmatic nerve, vagus, sympatic nerves).
Toxic effects are manifested in degeneration or even destruction of myelin membrane.
Axis cylinders undergo swelling and rarely necrosis.
Parenchymatous neuritis with dvelopment of the late paralysis of the palate, diaphragm,
and heart develops. The paralytic effects of diphtheritic neuropathy are often sharply
localized.
Paralysis of the voluntary muscles of the palate may produce a peculiar nasal quality of
the voice and a tendency to regurgitate fluids through the nose.
Paralysis of the diaphragm may lead to aspiration pneumonia.
Late paralysis of the heart may lead to acute cardiac failure.
Involvement of extraocular muscles may produce diplopia, and involvement of the ciliary
body may result in defective visual accommodation.
Clinically apparent weakness or paralysis of limbs is rare. Neuropathic manifestations of
diphtheria are usually temporary and disappear within 2 or 3 months if the patient
survives.
3. Hemorrhages, degeneration and necrosis of the cells are observed in the medullary layer of the
adrenal glands, foci of necrosis and disappearance of lipids are seen in the cortical layer. Acute
adrenal insufficiency may develop.
4. A nonspecific, nonsuppurative interstitial nephritis is frequent in diphtheria and is believed to be
responsible for the proteinuria often observed. Necrotic nephrosis and massive necroses of the
cortical layer in the severe cases of toxic diphtheria are observed in the kidneys. The renal lesion
usually resolves completely in patients who recover.
5. The liver is characteristically enlarged; hepatocytes exhibit cloudy swelling and less commonly
focal necrosis.
Diphtheria in the respiratory tract
Diphtheria of the respiratory tract is characterised by croupous inflammation of the
larynx, trachea, and bronchi with formation of fibrinous films, which can be discharged at
cough.
The epithelial surface becomes necrotic and easily adherent to the overlying membrane;
this adherence explains why raw bleeding, points are exposed when the membrane is
forcibly removed. If particularly extensive, the local process may produce mechanical
respiratory obstruction, stridor, and even asphyxia.
Croupous inflammation of the larynx in diphtheria is called true croup, propagation of
the process in the small branches of the bronchial tree is called descending croup, which
may be accompanied by development of focal pneumonia.
Complications in diphtheria of the respiratory tract are caused by
- Asphyxia due to obstruction of fibrinous films.
- Intubation or tracheotomy, which can result in decubitus.
- Secondary infection in decubitus causes purulent perichondritis of the cartilages of
the larynx, phlegmon, and purulent mediastinitis.
Death is caused by
- Asphyxia (spasm of the larynx in true croup or occlusion of the respiratory tract with
fibrinous films) or by accompanying pneumonia and purulent complications.
- Early cardioplegia in myocarditis and late cardioplegia or paralysis of the diaphragm
due to parenchymatous neuritis when antitoxic serum is not administered in time.
Scarlet fever (SF)
Scarlet fever is one of the forms of streptococcal infection, it is an acute infectious disease
accompanied by local inflammatory changes mainly in the pharynx and typical generalized rash. The
disease is common in children (3-12 years old), but it can also be observed in the adults.
177
Etiology and pathogenesis
The causative agent is beta-hemolytic streptococcal group A of different serological types.
The patients are infected by inhalation (air-droplets rout), but the disease can also be
transmitted through personal belongings and foodstuffs (mainly milk).
Incubation period is 3-7 days.
Pathogenesis of scarlet fever is complicated and explained by third factors: erythrogenic
toxin, microbial invasion and allergic reactions.
The duration of the disease is divided into two periods, toxic (first) and infectious allergic
(second) ones.
In the early stages there is rather severe pharyngitis and tonsillitis. These, combined with
fever, vomiting, and headache, make up the cardinal prodromal symptoms of scarlet
fever. Because there is no specific strain of beta-hemolytic streptococci responsible for
scarlet fever, bacteriologic studies do not provide a means for early diagnosis; in other
words, a diagnosis of throat infection caused by S. pyogenes is not a diagnosis of scarlet
fever. The diagnosis cannot be positively made until the second stage of the disease, which
is reached 1 to 5 days after the onset.
Morphology
1. Toxic period (1-2 weeks).
Local changes appear the inflammatory process in the site of the primary fixation (tonsils,
skin, lungs, seldom-genital tract), which is accompanied by regional lymphangitis and lymphadenitis.
This is called “primary scarlatinic affect” and “primary scarlatinic complex”. “Primary
scarlatinic affect” is characterized by catarrhal or necrotic tonsillitis.
Catarrhal tonsillitis (during the first few days) is manifested by hyperemia of pharynx
(“flaring pharynx” or “burning faucet”) with involvement to oral cavity and tongue.
It presents a “strawberry” appearance because of the erythematous papillae that project
from a gray-coated background. When peeling occurs, the tongue becomes beefy red and
glistening.
Necrotic tonsillitis is characterized by coagulative necrosis and ulceration.
Microscopically, there is a characteristic acute, edematous, neutrophilic inflammatory
reaction within the affected tissues. Necrosis may involve the soft palate, pharynx,
auditory tube (Eustaphian tube), middle ear; it can pass from the lymphatic nodes to the
subcutaneous fat of the neck. Rejection of the necrotic masses results in ulcers. Cervical
lymphatic nodes are plethoric, juicy, enlarged, with foci of necrosis and marked myeloid
infiltration.
General changes.
The general changes depending on toxemia are first of all rash.
A punctate erythematous rash that is most abundant over the trunk and inner aspects of
the arms and legs manifests exanthema. The face is also involved, but usually a small
area about the mouth (nasolabial triangle) remains relatively unaffected, to produce a
circumoral pallor.
Microscopically, there is a characteristic acute, edematous, neutrophilic inflammatory
reaction surrounding the affected tissues (skin and lymph nodes).
The inflammatory involvement of the epidermis is usually followed by hyperkeratosis of
the skin, which accounts for the scaling during defervescence. The hyperemia and
resultant red coloration of skin are manifestations of toxic injury (atony and dilatation) of
vascular endothelium. This hyperemia blanches on pressure and disappears on death;
thus little of the characteristic skin reaction is evident at autopsy.
3. The second period (allergic).
The second period may develop on the 3rd - 5th week of the disease rarely.
The second period begins with moderate catarrhal tonsillitis.
The most significant is development of acute or chronic glomerulonephritis with possible
nephrosclerosis development.
Skin rash, vasculitis, serous arthritis, verrucous endocarditis can be observed.
Complications are divisible into three major categories:
1 The results of bacterial disseminations locally – otitis media, sinusitis, cervical
adenitis, phlegmon of the neck, acute suppurative mastoiditis, and retropharyngeal
abscess.
178
2 The result of bacterial dissemination generally – metastatic foci of infection
throughout the body, or trunk septicemia.
3. The manifestation of extraordinary reactions to toxins (this may be brought about by
hypersensitivity) – interstitial nephritis or myocarditis, pericarditis, nonsuppurative
arthritis, and glomerulonephritis.
The death is caused by toxemia or septic complications.
Meningitis (M)
Meningitis (leptomeningitis) is an acute or chronic inflammatory process chiefly affecting
the pia and arachnoid mater, cerebrospinal fluid (CSF) and may be caused by bacteria,
fungi, or parasites.
It is usually caused by an infection, but chemical meningitis may also occur in response to
a nonbacterial irritant introduced into the subarachnoid space.
Infectious M. can be broadly classified as acute pyogenic (usually bacterial), aseptic
(usually viral), and chronic (bacterial or fungal).
Meningococcal infection is an acute infectious process which has three main forms:
nasopharyngitis, purulent meningitis and meningococcemia.
This is characterized by periodic epidemics, the disease is more common in children under
5 years, but the disease may occur in persons of any age.
The typical patient with acute pyogenic meningitis has general signs of infection with the
added symptoms and signs of meningeal irritation: headache, photophobia, irritability,
clouding of consciousness, and neck stiffness.
Etiology. The causative agent is meningococcus (Neisseria meningitidis), which
discharges the endotoxin.
Morphology
Meningococcal nasopharyngitis
It is characterized by catarrhal inflammation of the mucosa with marked hyperemia,
edema of the posterior wall of the pharynx and hyperplasia of lymphatic follicles.
This form is of great epidemiological importance as clinical diagnosis is often difficult.
Bacteriologic investigation is necessary for diagnose.
Meningococcal meningitis
It is characterized by the hyperemia of the pia mater, saturated with dull serous exudate
during the first days of the disease.
By the end of the 2nd - 3rd day the exudate becomes thicker, green-yellow, purulent. By
the 5th -6th day it becomes denser due to fibrinous effusion.
The process begins with basal surface and passes through, the perivenous spaces to the
convex surface mainly of anterior portion of the brain, locating there in the form of a
yellow-green “cap”.
The purulent process involves the meninges of the spine.
The meningeal vessels are enlarged and stand prominently.
The infection may extend into the ventricular system through the foramina of Magende
and Luschka causing ventriculitis.
Other complications of meningitis include hydrocephalus resulting from ventricular
obstruction or meningeal fibrosis, subdural effusion caused by fluid leading into the
subdural space through defects in the arachnoid and occuring most commonly in children,
and cranial nerve palsies probably related to inflammatory involvement of nerve roots
crossing the subarachnoid space.
The death may occur from the brain swelling with wedging of the cerebellum tonsils to the
great foramen and strangulation of the oblong brain during the acute period.
Later the cause of death is meningoencephalitis, purulent ependymitis or general cerebral
cachexia due to hydrocephalia and atrophy of the brain hemispheres during the following
periods.
Meningococcemia
Duration is 24-48 hours.
Bacteriemia and endotoxinemia lead to endotoxic shock with the development of
syndrome of disseminated intravascular coagulation.
179
Changes on the organs are characterized by generalized damage of microcirculation, skin
rash, changes in the joints, vascular membrane of the eyes, adrenal glands and kidneys.
Changes in the serous layers of the pericardium are observed.
The changes of the microcirculation are characterized by vasculitis and necrosis.
The rash is hemorrhagic, star-like, located mainly on the buttocks, lower extremities,
eyelids and scleras. There may be vesicles or dull dryish foci of necrosis in the centre of the
skin elements. Purulent arthritis is observed in the small joints of the extremities.
Focal necroses and hemorrhages or bilateral massive hemorrhages with the development
of acute adrenal insufficiency (Waterhouse-Friderichsen syndrome) are noted in the
adrenals.
Necrosis of nephrothelium of the tubules (necrotic nephrosis) is observed in the kidneys.
Serous meningitis and hemorrhage may occur.
The death of the patients is caused by bacterial shock, its severity is aggravated by
hemorrhages to the adrenals; acute renal insufficiency is not so common (in the adults).
When the duration of the disease is prolonged, the death occurs from septicemia or
purulent meningitis.
180
GASTROINTESTINAL INFECTIONS
Shigella bacillary dysentery (D)
Dysentery refers to diarrhea with abdominal cramping and tenesmus in which loose
stools contain blood, pus, and mucus.
Bacillary D. is caused by Shigella dysenteriae, S. flexneri, S. boydii, and S. sonnei as well
as certain O-type enterotoxic E.coli.
Transmission occurs by the fecal-oral route.
Symptoms appear 2 to 5 days after the ingestion of bacteria. The dose of organisms and
the status of host defenses influence the incubation period and severity.
The key to the pathogenicity of Shigella is its ability to invade and multiply in the
epithelium and lamina propria of the terminal ileum and colon, and destroy host cells.
Endotoxin probably adds to necrosis, but the role of enterotoxin produced by some species
of Shigella in the pathogenesis of dysentery is uncertain.
While clearly secondary to invasion, Shiga toxin probably contributes to the profuse
diarrhea that precedes dysentery in some patients.
This enterotoxin, which is related antigenically to the enterotoxin of enteropathogenic E.
coli, activates membrane-associated adenyl cyclase. Thus, shiga toxin, like cholera toxin
and E. coli enterotoxin, induces hypersecretion of fluid and electrolytes from the mucosa
of the terminal ileum. Water and electrolyte balance must be maintained to prevent
dehydration, prostration, and impaired mental status.
Morphology
Colitis has 4 stages:
1. Catarrhal colitis. The mucosa becomes edematous and hyperemic, and is covered by pus and mucus.
2. Fibrinous colitis. Within the course of 24 hours, a fibrinosuppurative exudate first patchily, then
diffusely covers the mucosa and produces a dirty gray-to-yellow pseudomembrane, consisting of
necrotic mucosa, neutrophils, fibrin, and erythrocytes. Sloughed pseudomembrane, together with
blood-tinged mucus, comprises the characteristic dysenteric stool of shigellosis.
3. Ulcer formation (ulcerative colitis).
4. Healing of the wound. The epithelium persists only in the depths of the crypts, and goblet cells
contain no mucus in the acute stage. Epithelial regeneration is rapid and healing is complete in 2
weeks.
Histologically, there is predominantly mononuclear leukocytic infiltrate within the lamina
propria, but the surfaces of the ulcers are covered with an acute, suppurative, neutrophilic
reaction accompanied by congestion, marked edema, fibrin deposition, and thromboses of
small vessels. As the disease progresses, the ulcer margins are transformed into active
granulation tissue. When the disease remits, this granulation tissue fills the defect, and the
ulcers heal by regeneration of the mucosal epithelium.
In case of solitary follicle cell hyperplasia, they enlarge and protrude over the surface of
the mucous membrane (follicular colitis and follicular-ulcerative colitis).
Lymphadenitis develops in the regional lymphatic nodes. Common changes are spleen
hyperplasia, fatty degeneration in the heart and liver, small-focal necroses in the liver,
necrosis of renal tubular epithelium.
Complications of dysentery are
- Perforation (microperforation) of the ulcer with development of. paraproctitis or
peritonitis, intestinal phlegmon.
- Intraintestinal hemorrhage
- Scar stenosis of the intestine is less common.
- Extraintestinal complications are bronchopneumonia, pyelonephritis, serous (toxic)
arthritis, and pylephlebilic abscesses of the liver, amyloidosis, intoxication, and
cachexia.
The death may cause by intestinal or extraintestinal complications.
Amebiasis
The protozoan parasite Entamoeba histolytica infects approximately 500 million persons in
developing countries. The disease is common in India, Mexico, and Colombia. Amebae cause
dysentery – bloody diarrhea, intestinal pain, fever – when they attach to the colonic epithelium, lyses
colonic epithelial cells, and invade the bowel well.
181
Morphology
Amebiasis most frequently involves the cecum and ascending colon, followed in order by
sigmoid, rectum, and appendix. In severe, full-blown cases, however, the entire colon is
involved.
Amebae invade the crypts of the colonic glands, burrow through the tunica propria, and
are halted by the muscularis mucosae. As the lesion progresses, the overlying surface
mucosa is deprived of its blood supply and sloughs. The earliest amebic lesions show
neutrophilic infiltrates in the mucosa, which later develop into ulcers that contain few host
inflammatory cells and areas of extensive liquefactive necrosis.
The mucosa between ulcers is often normal or midly inflamed.
In about 40% of patients with amebic dysentery, parasites penetrate portal vessels and
embolize to the liver to produce solitary, or less often multiple, discrete abscesses, some
exceeding 10 cm in diameter. Amebic liver abscesses have a scant inflammatory reaction
at their margins and a shaggy fibrin lining. Because of hemorrhage into the cavities, the
abscesses are sometimes filled with a chocolate-colored, odorless, pasty material likened
to anchovy paste. Secondary bacterial infection may make these abscesses purulent. As
the amebic abscesses enlarge, they produce pain by pressing on the liver capsule and can
be visualized with ultrasound. Amebic liver abscesses are treated with drainage and drugs
or with drugs alone
Rarely, amebic abscesses reach the lung and the heart by direct extension or appeared
through the blood into the kidneys and brain.
Salmonellosis and typhoid fever
Salmonellae are flagellated, gram-negative bacteria that cause a self-limited and waterborne gastroenteritis or a little-threatening systemic illness marked by fever.
Salmonellas invade nonphagocytotic interstitial epithelial cells as well as tissue
macrophages.
Typhoid fever
Typhoid fever (enteric fever) is an acute intestinal infectious disease caused by
Salmonella typhy abdominalis. Epidemics are possible but at present the disease is rare, its
course is not severe. The infection is parenteral.
The source of infection is a sick person or a human carrier whose excretions (faeces, urine,
sweat) contain the microbes.
Pathogenesis
The bacteria multiply in the lower portion of the small intestine and produce endotoxins.
On penetrating the intestinal mucosa, the organisms enter Peyer’s patches and solitary
follicles, quickly enter lymphatic vessels and mesenteric nodes, whence they reach the liver
and then, by the thoracic duct, the bloodstream. All this occurs in the incubation period,
usually 10 to 14 days. This is the first stage of the disease; in which generalization of the
infection occurs before localizing lesions draw attention to the intestine.
Bacteremia develops (1st week of the disease); the bacillus can be isolated from the blood
(homoculture). Bacteremia is associated with generalization of the infection.
Beginning with the 2nd week antibodies to the causative agent are determined in the
blood with agglutination reaction (Widal’s reaction).
Bacteremia is also associated with elimination the causative agent that is excreted with
the sweat, milk, urine, faeces, and bile. The patient is especially infective during this
period.
The most favourable conditions for the life of the bacteria are in the bile where they
intensively multiply (bacteriocholia).
They are excreted with the bile to the small intestine and cause hyperergic reaction in the
previously sensibilized lymphatic follicles. The condition results in necrosis of the intestine
lymphatic system.
Morphology
The changes in typhoid fever can be local and generalized.
182
Local changes occur in the mucous membrane and lymphatic system (group and solitary
follicles of the intestine). The most prominent changes develop in the Peyer's patches of the ileum
(ileotyphus).
These changes develop in 5 stages. Each stage takes approximately one week.
1. Medullar swelling is acute proliferative granulematous inflammation in lymphoid apparatus of
intestine with development macrophagal granulomas (“typhoid granuloma”). They consist of large
macrophages with pail-pink cytoplasm, containing bacteria. In mucosa the catarrhal inflammation is
found out. Proliferation of phagocytes with enlargement of reticuloendothelial and lymphoid tissues
throughout the body develop. Peyer’s patches in the terminal ileum become sharply delineated,
plateau-like elevations up to 1 cm in diameter, with enlargement of draining mesenteric lymph nodes.
Follicles are protruded in intestine lumen. Their surface is striated and like brain.
2. Necrosis. After 7 to 10 days, the picture in the intestine is complicated by necrosis and ulceration of
areas that formerly exhibited lymphoid hyperplasia.
3. Ulcer formation (“unclear ulcers”). In the second week, the mucosa over the swollen lymphoid
tissue is shed, resulting in oval ulcers with their long axes in the direction of bowel flow. In the colon,
ulcers are smaller and punctate, corresponding to the smaller lymphoid follicles there. Edges of ulcer
are irregular with necrotic tissue. Macrophages, lymphocytes and plasma cells, whereas neutrophils
are present near the ulcerated surface.
4. “Clean ulcer” has regular shape without necrotic tissue. In this stage the perphoration can develop.
5. Healing (recovery). Granulomas are sclerosed, necroses undergo to petrification.
General changes. The changes in typhoid fever may be typical only for this disease as well as
characteristic for any infection.
Roseolar-papular rash and typhoid granuloma in different organs occurs.
The latter are the processes in the organs of the lymphatic system and degenerative
changes in the parenchymal organs.
The spleen is enlarged, soft, and bulging, with uniformly pale red pulp, obliterated
follicular markings, and prominent sinus histiocytosis and reticuloendothelial
proliferation.
The liver shows small, randomly scattered foci of parenchymal necrosis in which the
hepatocytes are replaced by a phagocytic mononuclear cell aggregates, called “typhoid
nodule”.
These distinctive nodules also occur in the bone marrow and lymph nodes.
Gallbladder colonization, which may be associated with gallstones, causes a chronic
carrier that may require cholecystectomy to eliminate bacterial shedding.
Atypical forms are pneumotyphus, cholangiotyphus.
Complications
Intestinal (intraintestinal hemorrhages, ulcer perforation, peritonitis).
Extraintestinal (pneumonia, purulent perichondritis of the larynx, Zenker’ s necrosis of the
abdominal muscles, osteomyelitis, intramuscular abscesses).
The death is caused by the complications.
Salmonellosis
Salmonellosis is an intestinal infection caused by salmonellas. It is anthropozoonosis and
occurs both in human beings and animals.
The most often pathogenic organism is Salmonella typhi murium, salmonella enteritidis,
salmonella cholera suis.
Incubation’s period is 12-36 hours.
Clinical symptoms are accompanied with endotoxin and endotoxinemia: fever, diarrhea
and hypotony and endotoxic shock.
Pathology. Salmonellas cause three types of human disease (salmonellosis): interstitial
(toxic), septic, typhoid.
1. Interstitial salmonellosis (gastroenteritis) develops in food poisoning. It is
characterized by acute gastroenteritis causing severe-dehydration of the organism. The
disease resembles cholera that is why it is called “home cholera”.
2. Septic salmonellosis (septicemic diseases without specific organ-system localization)
differs from interstitial one in hematogenic generalization of the causative agent with
formation of metastatic abscesses in different organs while the changes in the small
intestine are not significantly pronounced.
3. Typhoid salmonellosis (specific enteric fevers) resembles typhoid fever.
183
Complications. Toxicoinfectious shock, purulent complications, dysbacteriosis when the
treatment is inadequate.
Cholera
Cholera is an acute gastrointestinal infectious quarantinic disease and is characterized by
diarrhea and exicosis.
The vibrios never invade the enteric epithelium but instead remain within the lumen and
secrete their endotoxin.
Secretory diarrhea is caused by released of an endotoxin, called cholera toxin, which is
nearly identical to E.coli endotoxin.
This is due to the exotoxin of the Vibrio cholera, which evokes an intense outpouring of
watery fluid and electrolytes into the gut lumen, resulting in severe diarrhea and
hypovolemic shok.
Vibrio cholera is comma-shaped, gram-negative bacteria that have been caused of seven
great long-lasting epidemics (pandemics) of diarrheal disease. Vibrio cholera locates in
water often.
The only significant natural reservoir of cholera appears to be humans, and the only
clinically significant portal of entry is the alimentary tract by the fecal-oral route. V.
cholera are appreciably sensitive to normal gastric acidity.
The incubation period is usually 1 to 5 days, after which a profuse watery diarrhea occurs
usually without tenesmus or abdominal distress.
Fluid loss can exceed 10 liters per day. Prostration is therefore rapid and profound.
The disease is ordinarily self-limited, with death or recovery occurring within a few days.
An asymptomatic convalescent carrier state is uncommon but can occur.
Drinking water contaminated with V. cholera and food prepared with contaminated
water is infectious. Those with a normal gastric acidity are much less susceptible than
those with low levels of stomach acid as a result of a gastrectomy or other cause. Vibrios
traverse the stomach, enter the small intestine and propagate.
Clinical-morphological stages of cholera
1. Choleric enteritis is characterized by the hard diarrhea. Morphologically: swelling of
enterocytes, serous edema of the intestine mucosa.
2. Choleric gastroenteritis is characterized by the hard diarrhea and vomit, increase of
dehydration. The loss of sodium and water causes severe diarrhea, called “rice-water stool”. Fluid
loss may exceed 1 liter per hour.
3. Choleric exicosis (algid):
Acute dehydration, hypovolemic shock, and metabolic acidosis follow quickly.
The patient exhibits dry skin, sunken eyes, lethargy, cyanosis, a weak pulse, faint heart
sounds, hemoconcentration, and elevation of serum proteins. The hematocrit may rise to
55-65 and the plasma specific gravity to 1.035-1.050. Patients are usually a febrile; body
temperature may be subnormal.
Rigor mortis develops quickly and persists for several days. The outlines of the muscles
are well pronounced (“gladiator posture”).
The skin is dry, creasy (especially on the fingers, “beef-steak hands”). Due to rapid
development of rigor mortis, resembles “goose’s skin”.
The mucous membranes, subcutaneous fat and muscles are dry; the muscles become dark
red. The blood in the veins is thick, dark. The serous membranes are also dry, covered
with sticky transparent mucus, which is stretched out in the form of threads.
Changes in different organs due to dehydration (spleen, liver, gallbladder, kidneys,
myocardium, brain) can be.
The spleen diminishes, its capsule becomes creasy, the follicles are atrophic, and pulp
hemosiderosis is observed.
Degeneration and focal necroses in the liver develop. Bile formation is disturbed. The
gallbladder is not distended, filled with clear light bile (“white bile”).
Necrotic nephrosis of the main portions of nephron (the changes observed in oliguria and
acute renal failure) is noted in the kidneys.
There are degenerative and necrobiotic changes in the brain and myocardium.
Treatment is prompt rehydration, and under such circumstances most patients survive.
After the onset of diarrhea, urine production ceases, but renal function improves when
184
fluid and electrolytes are replaced. Inadequate replacement, however, leads to prolonged
renal failure, with acute damage of tubules and the vacuolar lesions of hypokalemia.
Complications
There are nonspecific and unspecific complications of cholera. Cholera typhoid and postcholera uremia are specific complications. Nonspecific complications are pneumonia, abscesses,
phlegmon, erysipelas, and sepsis.
The death occurs in algid period and is caused by dehydration, coma, uremia, and
intoxication. At present owing to early adequate treatment (administration of water and salts,
antibiotics) the death rate has been considerably decreased.
Escherichia coli Infection
Escherichia coli, a gram-negative bacillus that is part of the intestinal flora, is also an
important opportunistic pathogen, causing diarrhea and dysentery, urinary tract infections,
pneumonia, and neonatal meningitis. E. coli causes at least three patterns of human enteric diseases:
enterotoxigenic, enteroinvasive, and enteroadherent.
1. Enterotoxigenic E. coli causes a diarrheal disease by elaborating two plasmid-mediated
enterotoxins. The heat-labile toxin is antigenically, structurally, and functionally related to the cholera
toxin, although the toxin of E. coli is less potent than that of cholera. As in cholera, the resulting
activation of adenylcyclase produces a hypersecretory diarrhea. The heat-stable toxin of E. coli is
different from cholera toxin and apparently acts to impair sodium and chloride absorption and to
reduce the motility of the small intestine. Dehydration and electrolyte imbalance is a significant cause
of morbidity and mortality when appropriate rehydration is lacking - a common combination among
infants in less developed countries. Enterotoxigenic E. coli is also responsible for 50% of traveller's
diarrhea.
2. Enteroinvasive E. coli produces a dysentery-like disease resembling shigellosis, although
it is less severe and requires a much larger infecting dose of organisms. Enteroinvasive E. coli invades
the intestinal mucosa and causes local tissue destruction and sloughing of necrotic mucosa. Bloody
mucoid stools contain neutrophils.
3. Enteroadhesive E. coli has only recently been associated with diarrheal diseases.
Enteroadhesiveness is plasmid-dependent and is apparently mediated by pili, which bind tightly to
receptors on the intestinal epithelial cells. The mechanism of diarrhea is unknown.
About 80% of all infections of the urinary tract in humans, ranging from mild cystitis to fatal
pyelonephritis, are caused by E. coli. In addition, E. coli is the etiologic agent in many cases of
nosocomial pneumonia, most often in elderly patients with underlying chronic disease. Aspirates of
endogenous oral flora containing E. coli appear to be the cause of this bronchopneumonia, although in
bacteremic patients pneumonia may result from seeding by septic emboli. Empyema is a common
complication, especially in patients with disease lasting more than a week.
Only rarely does E. coli cause meningitis in adults, but it is a major cause of neonatal
meningitis. Between 40% and 80% of infants with E. coli meningitis die, and the survivors frequently
suffer from neurologic or developmental anomalies.
185
TUBERCULOSIS
Tuberculosis is a chronic communicable disease with specific granulomatous
inflammation caused by a variety of tubercle bacilli, especially Micobacterium
tuberculosis hominis and M. t. bovis.
The organism is a strict aerobe and thrives best in tissues with high oxygen tension like in
the apex of the lung.
The lungs are the prime target, but any organ may be infected. The characteristic lesion is
a specifical granuloma with central caseous necrosis.
Tuberculosis still continues to be worldwide in distribution, more common in poorer
countries of Africa, Latin America and Asia. Other factors contributing to higher incidence
of tuberculosis are malnutrition, inadequate medical care, poverty, crowding, chronic
debilitating conditions like uncontrolled diabetes, alcoholism and immunocompromised
states like AIDS.
Mode of transmission
Human beings acquire infection with tubercle bacilli by one of the following routes:
By inhalation into the respiratory tract.
Ingestion. Through ingestion into GI tract leads to development to tonsillar or intestinal
tuberculosis.
Inoculation. Through mucous membranes of mouth and throat, skin.
Transplacental route results in development of congenital tuberculosis in fetus from
infected mother and is a rare mode of transmission.
Spread of tuberculosis
1. Local spread. This takes place by macrophages carrying the bacilli into the surrounding
tissues.
2. Lymphatic spread. Tuberculosis is primarily an infection of lymphoid tissues. Primary
complex is primary focus with lymphangitis and lymphadenitis.
3. Hematogenous spread. This occurs either as a result of tuberculous bacillemia because of
the drainage of lymphatics into the venous system or due to caseous material escaping through
ulcerated wall of a vein. This produces millet seed-sized lesions in different organs of the body like
lungs, liver, kidneys, bones and other tissues and is known as miliary tuberculosis.
4. By the natural passages.
Infection may spread from:
Lung lesions into pleura (tuberculous pleurisy).
Transbronchial spread into the adjacent lung segments.
Tuberculous salpingitis into peritoneal cavity (tuberculous peritonitis).
Infected sputum into larynx (tuberculous laryngitis).
Swallowing of infected sputum (ileocecal tuberculosis).
Renal lesions into ureter and down to trigone of bladder.
Hypersensitivity and immunity in tuberculosis
Hypersensitivity or allergy, and immunity or resistance, plays a major role in the
development of lesions in tuberculosis.
Tissue changes seen in tuberculosis are not the result of any exotoxin or endotoxin but are
instead the result of host response to the organism, which is in the form of development of
cell-mediated hypersensitivity (or type IV hypersensitivity) and immunity.
Tissue reaction to tubercle bacilli is different in healthy organism not previously infected
(primary infection) from an organism who is previously infected (secondary infection).
1. In the primary infection, intradermal injection of tubercle bacilli into the skin evokes
no visible reaction for 10-14 days. After this period, a nodule develops at the
inoculation site, which subsequently ulcerates and heals poorly. This process is a
manifestation of delayed type of hypersensitivity and is comparable to primary
tuberculosis in children.
2. In the secondary infection, the tubercle bacilli are injected into the skin who has been
infected with tuberculosis 4-6 weeks earlier. In 1 -2 days, the site of inoculation is
indurated and dark, attaining a diameter of about 1 cm. The skin lesion ulcerates which
186
heals quickly and the regional lymph nodes are not affected. This is called Koch’s
phenomenon and is indicative of hypersensitivity and immunity in the host.
Hypersensitivity and immunity are closely related and are initiated through T
lymphocytes sensitised against specific antigens in tuberculin.
Tuberculin (Mantoux) skin test. This test is done by intradermal injection of 0.1 ml of
tuberculoprotein, purified protein derivative (PPD). Delayed type of hypersensitivity
develops in individuals who are having or have been previously infected with tuberculous
infection which is identified as an indurated area of more than 15 mm in 72 hours.
However, patients having disseminated tuberculosis may show negative test due to
release of large amount of tuberculoproteins. A positive test is indicative of cell-mediated
hypersensitivity to tubercular antigens but does not distinguish between infection and
disease. The test may be false positive in atypical mycobacterial infection and false
negative in sarcoidosis, some viral infections, Hodgkin’s disease and fulminant
tuberculosis.
Immunisation against tuberculosis. Protective immunisation against tuberculosis is
induced by injection of attenuated strains of bovine type of tubercle bacilli, Bacilli
Calmette Guerin (BCG). Cell-mediated immunity with consequent delayed
hypersensitivity reaction develops with healing of the lesion, but the cell-mediated
immunity persists, rendering the host tuberculin-positive and hence immune.
Evolution of tubercule
The sequences of events, which take place when tubercle bacilli are introduced into the
tissue, are as under:
The inhaled organism enters the alveolus and is ingested by the alveolar macrophage. The
M. tuberculosis can either be killed by the macrophage; its growth inhibited or multiplies
inside the macrophage.
It behaves more like a parasite and lives in symbiosis with the cell.
The macrophages start phagocytosing the tubercle bacilli. In 2-3 days, the macrophages
undergo structural changes as a result of immune mechanisms - these modified
macrophages resemble epithelial cells and are called epithelioid cells.
The macrophages continue to enter the tissue either from circulating monocytes or from
local proliferation. Release of cytokines in response to sensitised CD 4 + T cells and some
constituents of mycobacterial cell wall play a role in formation of granuloma.
Some of the macrophages form multinucleated giant cells by fusion of adjacent cells.
The giant cells may be Langhans’ type or they may be foreign body type. The giant cells
may have 20 or more nuclei. These nuclei may be arranged at the periphery like horseshoe or ring or clustered at the two poles, or they may be present centrally (foreign body
giant cells).
Around the mass of epithelioid cells and giant cells is a zone of lymphocytes, plasma cells
and fibroblasts. The lesion at this stage is called hard tubercle due to absence of central
necrosis.
Within 10-14 days, the centre of the cellular mass begins to undergo caseation necrosis.
This stage is called soft tubercle, which is the hallmark of tuberculous lesions.
Acid-fast bacilli are difficult to find in these lesions and may be demonstrated at the
margins of recent necrotic foci and in the walls of the cavities.
In granuloma enclosed by fibrous tissue, calcium salts may get deposited in the caseous
material (dystrophic calcification) and sometimes the lesion may even get ossified over the
years.
Types of tuberculosis
I. Primary tuberculosis
II. Post primary tuberculosis
a) Secondary tuberculosis
b) Hematogenous tuberculosis
Primary Tuberculosis
The infection of an individual who has pot been previously infected or immunised is called
primary tuberculosis or Ghon’s complex or childhood tuberculosis.
187
Primary complex or Ghon’s complex is the lesion produced at the portal of entry with foci
in the draining lymphatic vessels and lymph nodes. Commonly involved tissues for
primary complex are lungs and hilar lymph nodes.
The incidence of disseminated form of progressive primary tuberculosis is particularly
high in immunocompromised host (in patients of AIDS).
The primary complex in lungs is located in the lower part of the right upper lobes or the
upper part of the lower lobes in 3, 8,9,10 segments usually. The initial infection produces
only slight abnormalities and may cause only slight malaise and mild fever.
Primary complex or Ghon’s complex in lungs consists of 3 components:
1. Pulmonary component (Primary affect or primary focus or Ghon’s focus) is 1-2 cm
solitary area of tuberculous pneumonia surrounding by perifocal serous inflammation.
It is located under the pleura, in the lower part of upper lobe and has white-yellow
color and firm consistence.
2. Lymphatic vessel component. The lymphatics draining the lung lesion contain
phagocytes containing bacilli and may develop beaded, miliary tubercles along the
path of hilar lymph nodes. Tuberculous lymphangitis the lymphostasis and tuberculi
along the edematous perivascular tissue occurs.
3. Lymph node component. This consists of enlarged hilar and tracheo-bronchial
lymph nodes in the area drained. The affected lymph nodes are matted and show
caseation necrosis.
In the case of primary tuberculosis of alimentary tract due to ingestion of tubercle bacilli,
a small primary focus is seen in the intestine with enlarged mesenteric Iymphnodes
producing tabes mesenterica. The enlarged and caseous mesenteric Iymphnodes may
rupture into peritoneal cavity and cause tuberculous peritonitis.
Fate of primary Tuberculosis
Primary complex may have one of the following sequelae:
I. Heal by fibrosis and in time undergo calcification and even ossification. In over 90% of normal adults
the infection follows this self-limited course, because the cellular immune response is sufficient to
control the multiplication of bacilli. Therefore, in both the lung and the lymph nodes the lesions of the
Ghon complex heal, undergoing shrinkage, fibrous scarring, and calcification. Most of the organisms
die, but a few remain viable for years. Later, if immune mechanisms wane or fail, the resting bacilli
may break out and cause serious tuberculous infection.
II. In some cases, the primary focus in the lung continues to grow called progressive primary
tuberculosis.
1. Growth of primary parenhymal injury
The primary Ghon focus in the lung is characterized by enlargement of caseous necrosis,
erodes the bronchial tree, and spreads, a sequence that results in adjacent “satellite”
lesions.
The lesion may enlarge in size and liquefy with a cavity formation (so called “primary
tuberculous caverna”) as in an adult or produce an area of consolidation. The caseous
material can enter into a bronchus and then spread to other parts of the lung or the
opposite lung, resulting in a tuberculous bronchopneumonia. When this happens the
caseous material is discharged leaving an acute cavity. It must be differentiated from lung
abscess caused by other conditions.
A subpleural focus can involve the pleura and cause pleuris followed by pleural effusion.
The infected material can by a retrograde spread, cause bronchial lesion and result in
endobronchial ulceration and stenosis, which can produce either a complete or partial
obstruction. This may lead to a segmental collapse, with compensatory emphysema or an
obstructive emphvsema. If the collapse persists for a long time, the affected lung may
become bronchiectactic.
2. Lymphogenous spreading
Lymphogenous spreading is characterized by involvement the new groups of lymph
nodes, such as: paratracheal, supraclavicular, subclavian, cervical and development of
tuberculous mezadenitis.
The enlargement of the bronchial lymph nodes may cause extrinsic compression on the
bronchus or erode into the adjacent structures. This leads to a variety of clinical
symptoms and pathological changes and form the spectrum of progressive primary
tuberculosis.
188
The effect of external compression of the Iymph nodes on the bronchus is similar to what
happens in the retrograde involvement from the parenchyma of the lung, complete or
partial obstruction.
The enlargement of the lymph nodes may produce a wheeze by compressing the bronchus.
The lymph node enlargement persists for a longer period and may cause further
lymphatic or hematogenous spread.
3. Hematogenous spreading
The most serious immediate complication is miliary tuberculosis, in which there is
invasion of the bloodstream by M. tuberculosis and dissemination throughout the body.
The name "miliary" derives from their supposed resemblance to millet seeds.
This occurs when the parenchymal part of the Ghon complex involves a pulmonary artery
or vein and discharges its infected contents into the blood.
Multiple minute granulomas develop in many organs of the body. The lesions are classically
0.5 mm to 2 mm in diameter, yellowish white, and evenly distributed through the affected
organ. A punctate area of necrosis may be seen in the center.
Microscopically, the lesions of miliary tuberculosis consist of small granulomas, usually
with a central necrotic portion in which numerous organisms are seen.
Few organs are spared; those most often involved are the lung (mainly by recirculation of
the organisms), spleen, liver, kidney, meninges, and bone marrow.
Miliary tuberculosis used to be found most often in young children, but in industrialized
countries it has become more common in the elderly and debilitated, in alcoholics, and in
high-risk racial groups.
Postprimary Tuberculosis
Hematogenous Tuberculosis
The healed lesions of primary tuberculosis may get reactivated. The bacilli lying dormant in
acellular caseous material are activated and cause progressive hematogenous tuberculosis.
Hematogenous tuberculosis appears after primary tuberculosis under following conditions:
The presence of sensibilization to tuberculin.
Strongly pronounced immunity.
The presence of foci is healed after hematogenous generalization of primary tuberculosis
(sifting).
Hematogenous tuberculosis is characterized by proliferative reaction or formation of the
granulomas and hematogenous spreading.
Classification of Hematogenous tuberculosis
1. Generalized hematogenous tuberculosis is more serious form with dessimenation of granuloms:
а) The most acute tubercular sepsis.
b) Acute general miliary tuberculosis.
c) Acute general large-focal tuberculosis.
d) Chronic miliary tuberculosis.
2. Hematogenous pulmonary tuberculosis
а) Acute miliary tuberculosis.
b) Chronic miliary tuberculosis.
c) Chronic large-focal tuberculosis or hematogenous-disseminative.
Features of hematogenous-disseminative tuberculosis:
May by in adults only.
Prevalence apex- plural localization.
Proliferative tissue reaction.
Development of the pneumosclerosis and emphysema of lungs.
Cor pulmonale (hypertrophy of right ventricle of heart).
Presence of unpulmonary tubercular foci.
3. Hematogenous tuberculosis with unpulmonary lesions or organic tuberculosis is characterized
by acute and chronic destruction and insufficiency of organs. It may be
Bone- articular. In tuberculosis of bones and cartilages tuberculous osteomyelitisespccially spondylitis, coxitis (hip joint disease), gonitis develop.
Tuberculosis of the kidneys.
Tuberculosis of genital tract.
189
Tuberculosis of skin.
Tuberculosis of endocrine organs and others.
Tuberculosis of the brain is most important hematogenous localization. May cause
meningitis or abscess. Meningitis is characterized by numerous granulomas in the
leptomeninges, with features of chronic meningitis. Infection is most marked around the
base of the brain and, even when infection is treated, there is often development of
meningeal fibrosis to cause hydrocephalus. Tuberculous abscess, (tuberculoma) forms
with infection of the brain parenchyma. A tuberculoma is typically a firm, lobulated mass
of granulomatous inflammation with central caseous necrosis, up to several centimeters
in diameter, and walled off by fibrous tissue. Lesions occur within the cerebral
hemispheres, but are most common in the cerebellum. Treatment with antibiotics is
usually ineffective and surgical excision is required.
Secondary Tuberculosis
Secondary tuberculosis usually results from reactivation of dormant, endogenous tubercle
bacilli in a sensitized patient who has had previous contact with the tubercle bacillus. In
some cases, the disease is caused by reinfection with exogenous bacilli.
Secondary tuberculosis may develop any time after primary infection, even decades later.
Reactivation typically begins in the apical or posterior segments (often 1-st and 2-nd
segments) of one or both upper lobes (“Simon’s foci”), where the organisms were seeded
during the primary infection. Only Pulmonary localization takes place.
Contact and intracanalicular spreading.
Shifts of the clinical-morphological forms.
The symptoms of secondary tuberculosis begin with cough, which may be erroneously
attributed to smoking or to a “cold”. Low-grade fever develops, with general malaise, fatigue,
anorexia, weight loss, and often night sweats. As the disease progresses, the cough worsen and the
sputum may be streaked with blood. The rupture of a branch of the pulmonary artery in the wall of a
cavity leads to massive hemoptysis and asphyxiation or exsanguination.
Forms or stages of the secondary tuberculosis
1. Acute local tuberculosis is characterized by specific endo-, meso--, and panbronchitis.
During the treatment the exudative process is replaced by proliferative process. Foci of caseous
necrosis are incapsulated and petrificated.
2. Fibrous-local tuberculosis forms due to intensification of acute local tuberculosis with
formation of fibrous capsule.
3. Infiltrative tuberculosis is characterized by extension of perifocal inflammation.
4. Tuberculoma consists of focus necrosis surrauded by fibrous capcule. Size of tuberculoma
may be near 2-5 cm. It must be differentiated from tumor of the lungs.
5. Caseous pneumonia develops due to progression of infiltrative tuberculosis. The caseous
material from a case of secondary tuberculosis in an individual with high degree of hypersensitivity
may spread to rest of the lung producing caseous pneumonia. The caseous changes prevail over
perifocal inflammation.
6. Acute cavernous tuberculosis develops due to lyses of caseous necrosis and
characterized by formation of the round cavity. It must be differentiated from primary cavernous
tuberculosis.
7. Fibrous – cavernous tuberculosis is most frequent form. Macroscopically, the lesions
are spherical and cavitary - the so-called coin lesions. A fibrous capsule surrounds a caseous, acellular
center, which contains numerous tubercle bacilli. From these cavitary nodules the organisms can
spread through the lungs and be discharged into the air during bouts of coughing. Microscopically,
the wall of cavity shows eosinophilic, granular, caseous material, which may show foci of dystrophic
calcification. Widespread coalesced tuberculous granulomas composed of epithelioid cells; Langhans’
giant cells and peripheral mantle of lymphocytes and having central caseation necrosis are seen. The
cuter wall of cavity shows fibrosis.
The wall of cavern has three membranes:
Internal membrane occurs by necrotic tissue.
Medium membrane occurs by special granular tissue.
External membrane occurs by fibrous tissue.
Internal surface may be to connect with bronchus; therefore process spreads along bronchi
into others sites of the lungs.
Complications of cavitary secondary tuberculosis
190
Aneurysms of patent arteries crossing the cavity producing hemoptysis.
Extension to pleura producing bronchopleural fistula.
Tuberculous empyema from deposition of caseous material on the pleural surface.
Thickened pleura from adhesions of parietal pleura.
8.Cirrhotic tuberculosis is a progressive variant of fibrous – cavernous tuberculosis. Lungs
are deformed due to development of the diffuse pneumosclerosis.
These pulmonary lesions of secondary tuberculosis are often complicated by a
variety of secondary effects, including
1. Scarring and calcification.
2. Spread to other areas.
3. Pneumothorax, pleural fibrosis and adhesions, with associated pleurisy, sharp pleuritic pain,
and shortness of breath.
4. Rupture of a caseous lesion, which spills bacilli into the pleural cavity.
5. Erosion into a bronchus, which seeds the mucosal lining of bronchioles, bronchi, and
trachea.
6. Implantation of bacilli in the larynx, which causes laryngitis, hoarseness, and pain on
swallowing. Lesions of secondary tuberculosis acquired through the gastrointestinal tract (usually
with M. t. bovis) can lead to entrapment of bacilli in lymphoid patches of small and large bowel.
Causes of death
Chronic respiratory-cardiac insufficiency due to development cor pulmonale.
Acute hemorrhage due to arrosion of vessels.
Chronic renal insufficiency due to development of amiloidosis of kidneys.
Due to intoxication and sepsis.
191
SYPHILIS
Syphilis (lues) is a sexually transmitted disease of mankind caused by the spirochette
Treponema pallidum.
Stages of syphilis:
1. Primary (the chancre).
2. Secondary (disseminated).
3. Tertiary (with lesions of deep organs following a latent period of 2 to 20 years or
more).
The chancre develops at the site of inoculation in 10 to 90 days (average 21 days) and has a
characteristic “luetic vasculitis”, in which endothelial cells proliferate and swell, and the walls of the
vessels become thickened by lymphocytes and fibrous tissue.
Morphology
In primary Syphilis, the chancre is a slightly elevated, firm, reddened papule, up to
several centimeters in diameter that erodes to create a clean-based, shallow ulcer. Histologically, the
chancre contains an intense infiltrate of plasma cells, with scattered macrophages and lymphocytes
and an obliterative endarteritis. The regional nodes are usually enlarged and may show nonspecific
acute or chronic lymphadenitis, plasma cell-rich infiltrates, or focal epithelioid granulomas. The
combination of chancre, lymphangitis, and lymphadenitis is called primary syphilitic complex.
Secondary Syphilis. It presents as a widespread skin rash (pox) of varying appearance,
ulceration of mucous membranes, generalized lymphadenopathy, damage to various individual organs
and tissues. There are constitutional effects – particularly fiver and anemia.
The essential pathology is the presence of very numerous spirochaetes accompanied by focal
infiltration of lymphocytes, plasma cells and macrophages with mild arteritis. Infectivity is very high.
Tissue destruction is minimal and healing occurs without scarring. A latent stage of long duration is
followed in 35% of cases by tertiary syphilis.
Tertiary (Late) Syphilis. The lesions, which may occur at any time for many years after
healing of the secondary phase, offer striking contrasts. This stage is characterized mainly by local
destructive lesions, the result of cell-mediated immune reactions (T-cells) causing necrosis of tissue. It
occurs years after the initial infection and most frequently involves the aorta, the central nervous
system, and the liver, bones and testes (gummas).
The main forms are:
1. Gumma.
This is a localized area of necrosis, which may affect large parts of any organ or tissue but
particularly bones, testis and liver and looks like white-gray and rubbery formation.
In the liver, gumma may produce the coarsely nodular pattern of cirrhosis, termed hepar
lobatum because of the simulation by the deep scars of multiple lobes.
Bone and joint gummas lead to areas of cortical and articular destruction. Pathologic
features and joint immobilization may result.
Testicular gummas often cause painless enlargement of the affected testis, thus simulating
a tumor
Histologically, the gummas contain a center of coagulated, necrotic material and margins
composed of plump or palisaded macrophages and fibroblasts surrounded by large
numbers of mononuclear leukocytes, chiefly plasma cells.
2. Syphilitic aortitis. The aorta is affected by an infiltration of lymphocytes and plasma cells
beginning around the vasa vasorum and extending into the media, causing weakening due to focal
destruction (windowing) of the specialized elastic tissues. There is compensatory irregular thickening
of the intima (tree-bark appearance), but the important effect is expanding aneurysm formation.
3. Neurological Syphilis. Neurosyphilis takes one of several forms, designated meningovascular
Syphilis, tabes dorsalis, and general paresis.
Meningovascular – mainly affects the meningeal blood vessels and causes neurological
impairment secondary
Parenchymatous:
a) General paralysis of the insane – severe destruction of cerebral tissue, atrophy of
convolutions, enlargement of ventricales;
b) Tabes dorsales – the damage specifically affected the posterior roots and columns of
spinal cord – is associated with characteristic clinical symptoms due to lose of
proprioceptive sensation in the legs.
192
Congential syphilis is most severe when the mother’s infection is recent. In perinatal and
infantile Syphilis, a diffuse rash develops. Syphilitic osteochondritis and periostitis affect all bones.
Destruction of the vomer causes collapse of the bridge of the nose and, later on, the characteristic
saddle nose deformity. Periostitis of the tibia leads to excessive new bone growth on the anterior
surfaces and anterior bowing, or saber shin. The liver is often affected severely in congenital syphilis.
Diffuse fibrosis permeates lobules to isolate hepatic cells into small nests. Gummas are occasionally
found in the liver, even in early cases. The lungs may be affected by a diffuse interstitial fibrosis.
The late-occurring form of congenital syphilis is distinctive for the triad of interstitial
keratitis, Hutchinson's teeth, and eight-nerve deafness.
SEPSIS
Sepsis is general infectious disease caused by infections getting into the organism and differs
from other infectious diseases.
Sepsis is severe disease with high lethality. The death rate in sepsis is very high. The
incidence of sepsis has increased recently which is associated with the appearance of
antibiotic-resistant strains of bacteria and administration of cytostatic preparations
causing immune system insufficiency.
Epidemiological feature is polyetiology (except viruses), not infectious illness. Sepsis may
be cause by different causative agents (staphylococci, streptococci, pneumococci,
meningococci, blue pus bacilli, tuberculosis mycobacteria, typhoid bacilli, fungi and other
agents, except for viruses).
Sepsis is not contagious; it cannot be reproduced experimentally.
Clinical features - irrespective of the character of the activator displays of illness are
stereotyped, are stipulated by generalization of infection and inadequate reaction of
organism on the infection.
The course of the disease is not cyclic, as it is observed in many infections.
There is no certain incubate period. The duration of the disease is different (from some
days to several months and even years), that is why some forms of the disease may be
defined, i.e. very acute, acute, subacute, and chronic.
Immunologic peculiarity of the sepsis is that immunity is not formed at this disease;
inadequate reaction on the activator develops, hyperergic reaction prevails.
Morphological feature is the fact that the local and general changes have no specific
features as it is observed in many infections.
Pathogenesis
Sepsis is a special form of interaction of macro- and microorganism, significance of which
is equivalent.
Hyperergic reaction of the organism on infects and absence of immunity stimulates
generalization of infection, acyclic course, prevalence of general reaction and losses of the
ability to locate infection.
Morphology
1. Local changes.
Local changes occur by the primary focus of infection (portal of entry) or at some
distance, in some cases it is absent.
Usually it is a focus of purulent inflammation, sometimes with no changes.
The infection propagates from the focus through the lymph and blood vessels.
Lymphangitis, lymphothrombosis and lymphadenitis, but also phlebitis and
thrombophlebitis quickly develop.
There is purulent thrombophlebitis, progressing to thrombobacterial embolism.
2. General changes
General changes at sepsis have degenerative, inflammatory and hyperplastic character.
Degenerative changes develop in parenchymatous organs and often finish by the necrosis.
The inflammatory processes in parenchymatous organs and vessels occur.
Inflammatory changes are represented by interstitial septic nephritis, hepatitis,
myocarditis, and acute polypous-ulcerative endocarditis with the tissue melting and
tearing off of the valve.
Vasculitis, intoxication, increasing of vascular permeability, anemia stimulates the
hemorrhagic syndrome.
193
Hyperplastic processes develop in blood-creating and lymphatic tissues.
Hyperplastic processes in sepsis are observed mainly in the hemopoietic and lymphoid
tissue.
Bone marrow hyperplasia occurs in the flat bones. The yellow bone marrow of the tubular
bones becomes red.
In blood leukocytosis and, sometimes, immature leukocytes are found, the so-called
leukemoid reaction develops.
Peripheral lymphonodes are increased; spleen is acutely increased, flabby on cut and of
red color. Spleen produces large scrap of pulp (“septic splenitis”).
Hyperplastic processes in histiocyte-macrophage system are the cause of the liver
enlargement.
Hemolytic jaundice may result from hemolytic action of some bacterial toxins.
Classification of sepsis
A number of features are taken into account in classification.
I. According to the etiology: staphylococcal, blue pus bacillus and association of these
microorganisms, meningococcal, pneumococcal, gonococcal, colibacillary, anthracic, tuberculous.
II. According to portal of entry of infectious agent (location of the septic focus).
Therapeutic (parainfectious).
Tonsilogenic sepsis.
Surgical.
Uterine.
Otogenic.
Odontogenic.
Umbilical.
Pulmonary.
Cryptogenous (portal of entry of infectious agent is absent).
III. According to the clinical-morphologic forms of sepsis: septicemia, septicopyemia, septic
endocarditis and chronic septicemia.
Septicemia
It is a form of sepsis, for which toxicosis (high temperature, delirium) are characteristic,
increased reactivity of organism (hyperergia), absence of purulent metastases and rapid
course.
The etiology is frequently streptococcus.
Primary septic focus is frequently absent.
The skin and sclera are usually yellow (hemolytic jaundice).
Hemorrhagic syndrome is well pronounced (petechial rash, hemorrhages to the serous
and mucous membranes and internal organs).
Hyperplasia of lymphoid and hemopoietic system is typical: the spleen is enlarged, with
pulp scraping (“septic spleen”). The lymph nodes are also enlarged.
Proliferation of lymphoid and reticular cells as well as accumulation of mature and
immature blood cells are found in the spleen and lymph nodes.
Increased hemopoiesis with formation of a large number of immature forms is noted in
the bone marrow of the flat bones and in the diaphyses of the bones.
The foci of extramedullar hemopoiesis appear.
Interstitial inflammation develops in the parenchymal organs (heart, liver, kidneys). The
stroma of the organs is edematous; infiltration by neutrophils, lymphocytes, and
histiocytes is noted.
Septicemia is also characterized by increased vascular permeability, fibrinoid changes in
the vessels, allergic vasculitis that is responsible for hemorrhagic syndrome.
Septicopyemia
It is the form of sepsis, main attributes of which are purulent processes in the entrance of
infection and bacterial embolism with formation of abscesses in many organs and tissues.
In contrast to septicemia, hyperergy signs are moderate; the course of the disease is not
very acute.
The development is assosiated with staphylococcus and blue pus bacillus.
At the dissection there is primary septic focus, it is usual in the entrance of infection with
purulent lymphangitis and lymphadenitis.
194
The purulent thrombophlebitis in the primary septic focus is a source of thrombobacterial
embolism, which causes the creation of metastatic abscesses in organs.
At first metastatic abscesses appear in the lungs, then in the liver, kidneys (apostematous
nephritis), subcutaneous fat, bone marrow (purulent osteomyelitis), synovial membranes
(purulent arthritis), the heart valves (acute septic polypous-ulcerative endocarditis).
Besides, purulent pleuritis and pericarditis develop in the cases of lung abscess. In liver
abscess, purulent peritonitis develops. Kidney abscesses are complicated with peri- and
paranephritis; skin abscess is complicated with phlegmon.
Hyperplastic processes in blood-creating lymphatic tissue are expressed more poorly. The
lymphatic nodes are not increased.
Spleen is septic.
Interstitial inflammation in parenchymatous organs is moderate or is absent.
Septic (bacterial) endocarditis
It is the form of sepsis, for which septic lesion of valves of the heart is characteristic.
Hyperergia occurs and it can be considered to be bacterial septicemia.
The presence of primary septic focus on valves of the heart stimulates hyperergic damage
of cardiac - vascular system.
The most often causative agents are staphylococcus albus, aureus, streptococcus viridian,
and enterococcus.
In the basis of hyperergia reactions of hypersensitivity lays, stimulated by toxic immune
complexes circulating in the blood, containing antigen of activator and causing to
generalized vasculitis.
Increasing of vascular pemerability, thromboembolic syndrome, cellular reactions of
stroma are marked.
Classification
According to the character of course:
Acute (about 2 weeks).
Subacute (till 3 months).
Chronic (months and years).
Depending on the presence of the background disease, septic endocarditis (especially subacute and
acute) is divided into 2 types:
On unchanged valves (intact valves) -primary septic endocarditis (Chernogybov’s
disease), in 20- 30 % of cases.
Developed on changed valves (defective) - secondary septic endocarditis in 70-80 % of
cases.
Morphology
Polypous-ulcerative endocarditis develops on both sclerotic and intact valves.
Large thromboembolic polyp-shaped plaques appear on sclerotic valves.
The plaques are easily crumbled and are saturated with calcium, which is characteristic
for the disease.
After removal of the plaques, ulcerative defects are seen in the sclerotic and deformed
cusps of the valves.
Thrombotic plaques are located not only on the cusps but also on the parietal
endocardium.
When the aortic valves are injured, the disease involves the aortic intima.
The spleen is enlarged due to prolonged pulp hyperplasia; there are infarcts in the organ.
Immune-complex diffuse glomerulonephritis develops in the kidneys. Infarctions and
postinfarction scars are frequently observed.
Interstitial inflammatory processes, vasculitis, hemorrhages, infarctions are observed in
different organs.
The foci of softening and hemorrhages are observed in the brain due to vascular changes
(vasculitis, aneurysm) and thromboembolism.
The so-called peripheral signs of septic endocarditis are
a) Petechial hemorrhages in the conjunctiva near the internal angle of the lower eyelid
(“Lukin-Libman spots”).
b) Nodular thickening on the palm surface of the hand (“Osler’s nodes”).
c) Thickening of the nail phalanges (“drum sticks”).
195
d) Necrotic foci in the subcutaneous fat.
e) Hemorrhages to the skin and subcutaneous fat (Jeinway’s spots).
f) Jaundice.
Thromboembolic complications are frequent, as the source of thromboembolism;
thromboendocarditis is most commonly localized in the left heart.
Thromboembolism frequently becomes generalized and dominates in the clinical picture
of the disease.
The embolisms give the rise to infarctions in the lungs, spleen, kidneys, retina, and skin
necrosis, gangrene of the extremities, intestine, foci of softening in the brain.
In spite of the presence of streptococci in the thrombi, suppuration in the tissue is absent
which suggests hyperergic reaction of the organism in septic endocarditis.
Chronic septicemia
This form of sepsis is characterised by durably availability, not healing primary septic
focus.
These septic foci can be found in carious teeth, tonsils but more frequently they are large
suppurations resulting from wounds.
Extensive purulent processes, causing to intoxication, progressing exhaustion (cachexia)
and amyloidosis take place.
In organs and tissues there is atrophy, dehydration are expressed.
Brown atrophy is found in the liver, myocardium, and striated muscles.
The spleen is decreased.
Septic shock
Septic shock is currently the most common cause of death in intensive care units.
It results from the spread of microbes from severe localized infections (e.g., abscess,
peritonitis, pneumonia) into the bloodstream.
The majority of cases are caused by endotoxin-producing gram-negative bacilli - E.coli,
Klebsiella pneumonia, Proteus species, Pseudomonas aeruginosa, Serratia, and
Bacteroides - hence the term endotoxic shock.
Endotoxins are bacterial wall polysaccharides, consisting of a toxic lipid A core
component and a complex polysaccharide coat. Gram-positive cocci, such as pneumococci
and streptococci, and certain fungi, as well as gram-positive bacterial toxins produce a
similar syndrome.
Shock is a progressive disorder that may lead to death.
Shock tends to evolve through three stages:
1. An initial nonprogressive phase during which reflex compensatory mechanisms are
activated and perfusion of the vital organs is preserved.
2. A progressive stage characterized by tissue hypoperfusion and onset of an everwidening circle of circulatory and metabolic imbalances.
3. In finally, an irreversible stage that sets in after the body has incurred cellular and
tissue injury so severe that even if therapy corrects the hemodynamic defects survival
is not possible.
Morphology
These reactive features are nonspecific and are present in most bacterial septicemias.
Shock is characterized by hypoxic failure of multiple organ systems, and hence the cellular
changes may appear in any tissue. They are particularly evident in the brain, heart,
lungs, kidneys, liver, spleen, adrenals and gastrointestinal tract.
In the brain the so-called ischemic encephalopathy may develop.
The heart may undergo a variety of changes. Subendocardial hemorrhages and necrosis,
or “zonal lesions”, sometimes appear in all forms of shock. The term zonal lesions refers to
apparent hypercontraction of a myocyte, including shortening and scalloping of the
sarcomere, fragmentation of the Z band, distortion of the myofilaments, and displacement
of the mitochondria away from the intercalated disc.
The kidneys may be severely affected in shock, and that is why oliguria, anuria, and
electrolyte disturbances constitute major clinical problems. The renal changes are
referred to as acute tubular necrosis.
The lungs are seldom affected in pure hypovolemic shock because they are resistant to
hypoxic injury, but when the vascular collapse is caused by bacterial sepsis or trauma,
196
changes may appear that are referred to as “shock lung”. They are referred to as the acute
respiratory distress syndrome.
Splenomegaly of moderate degree (250 to 350 g) is common in acute systemic infections
and is referred to as “acute reactive hyperplasia” or “septic splenitis”. The spleen is
enlarged and soft, and the cut surface demonstrates an equal prominence of the red and
white pulp. Lymphoid hyperplasia with germinal center formation is pronounced, and
plasma cell hyperplasia is present in the marginal zone of the white pulp and in the cords.
Histiocytic hyperplasia is equally prominent.
The abscesses of the liver may take place also.
The adrenal alterations encountered in shock comprise in essence those common to all
forms of stress and so might be referred to as “ the stress response”.
The gastrointestinal tract may suffer patchy mucosal hemorrhages and necroses
referred to as “hemorrhagic enteropathy”.
Virtually all of these organs changes may revert to normal if the patient survives.
However, loss of neurons from the brain and of the myocytes from the heart is, of course,
irreversible. However, most patients who suffer shock so severe as to produce irreversible
changes succumb before these alterations become well developed.
It is evident that postshock course of the patient does not lack for threats to life. The
prognosis varies with the origin of shock and its duration.
197
LITERATURE
1. Anderson’s Pathology // Edited by John M. Kissane. The C.V. Mosby Company. –
Toronto – Philadelphia, 1990. – 980 p.
2. Dacie JV, Lewis SM: Practical Haematology, 7th ed. London, Churchill Livingstone,
1991.
3. Heptinstall RH: Pathology of the Kidney (3 vols). London, Little, Brown and Company,
1992.
4. Kumar V, Cotran RS, Robbins SL: Basic Pathology, 6th ed. Philadelphia, WB
Saunders company, 1997.
5. Ramzi S. Kotran, Vinay Kumar, Stanley S. Robbins. Robbins Pathologic Basis of
Disease, W.B. Saunders Company, USA, 1994 - 1400 p.
6. Rosai J: Ackerman's Surgical Pathology (2 vols), 8th ed. Mosby, St Louis, 1999.
7. Rubin E, Farber JL: Pathology, 3rd ed. Philadelphia, JB Lippincott Company, 1999.
8. Stevens A, Lowe J: Pathology, 1st ed. London, Mosby, 1995.
9. Sorokina I.V., Yakovtsova A.F. Lectures in Pathological anatomy. – Kharkiv: Tornado,
2000.-254p.
10. Thomas C. Macropathology. – B.C. Decker Inc. - Toronto – Philadelphia, 1990. –355
p.
11. Thomas C. Histopathology. – B.C. Decker Inc. - Toronto – Philadelphia, 1989. – 386 p.
12. Zagorulko A.K. Short lectures on pathology (pathological anatomy). – Simferopol: 2
ed. CSMU, 2002 - 222 p.
13. Струков А.И., Серов В.В. Патологическая анатомия. – М.: Медицина, 1993. – 687
с.
14. Серов В.В., Пальцев М.А., Ганзен Т.Н. Руководство к практическим занятиям по
патологической анатомии. - М.: Медицина, 1998. – 544 с.
15. Серов В.В., Пальцев М.А. Патологическая анатомия. Курс лекций. Учебное
пособие. - М.: Медицина, 1998. – 640 с.
198
CONTENTS
PART I. GENERAL PATHOLOGY
I. INTRODUCTION ON PATHOLOGY
CELLULAR INJURY AND CELLULAR DEATH
II. INTRACELLULAR ACCUMULATIONS (PARENCHYMAL DEGENERATIONS OR
DYSTROPHIES)
III. EXTRACELLULAR ACCUMULATIONS (MESENCHYMAL DEGENERATIONS)
IV. PATHOLOGY OF PIGMENTS. Mineral metabolism disturbance
V. IRREVERSIBLE CELLULAR INJURY: necrosis and apoptosis
VI.CELLULAR ADAPTATIONS:
Atrophy Hypertrophy and hyperplasia
Metaplasia
Dysplasia
Healing
Repair
VII. HEMODYNAMIC DISTURBANCES:
Hyperemia and congestion
Hemorrhage
Ischemia
Infarction
Stasis
Thrombosis
Embolism
Shock
Disseminated intravascular coagulation
Edema
VIII. INFLAMMATION:
Acute inflammation
CHRONIC INFLAMMATION
SYPHILIS
IX. IMMUNOPATHOLOGY
X. NEOPLASIA
(General pathomorphology of neoplasia)
XI. EPITHELIAL TUMORS
XII. THE MOST OFTEN TUMORS
XIII. MESENCHYMAL TUMORS
XIV. TUMORS OF NERVOUS SYSTEM AND BRAIN MEMBRANES
XV. TUMORS OF MELANIN-PRODUCING TISSUE
XVI. TUMOROUS DISEASES OF BLOOD AND LYMPHATIC SYSTEMS:
Leukemias
Lymphomas
PART II. SYSTEMIC PATHOLOGY
I. DISEASES OF BLOOD AND LYMPHATIC SYSTEMS:
199
Anemias
II. DISEASES OF CARDIOVASCULAR SYSTEM:
Atherosklerosis
Hypertension
Ischemic heart disease: acute and chronic
III. RHEUMATIC DISEASES:
Rheumatic fever (RF) and Rheumatic heart disease (RHD)
Rheumatoid Arthritis
Systemic Lupus Erythematosus (SLE)
Bechterew's disease
Systemic scleroderma
Dermatomyositis
IV. DISEASES OF RESPIRATORY SYSTEM:
ACUTE BACTERIAL INFECTIONS OF THE LUNGS: Pneumonias
CHRONIC OBSTRUCTIVE PULMONARY DISEASE:
Chronic Bronchitis
Bronchiectasis (BE)
Emphysema
Bronchial Asthma (BA)
Chronic Lung Abscess (LA)
Idiopathic Pulmonary Fibrosis
V. DISEASES OF ALIMENTARY SYSTEMS
Tonsillitis
Gastritis
Peptic Ulcer Disease
Appendicitis
VI. DISEASES OF THE LIVER:
Hepatosis
Viral hepatitis
Cirrhosis of Liver
Alcoholic liver disease
Cholelitiasis (Gallstones)
Cholecyscitis
Pancreatitis
VII. DISEASES OF KIDNEY AND URINARY TRACT
Glomerular Diseases
Nephrotic Syndrome
Tubulopathy
Acute Tubular Necrosis
Tubulointerstitial Disease
Pyelonephritis
Urolitiasis
Hydronephrosis
Cystic Disease of kidneys
Chronic renal failure
VIII. GENITAL TPACT DISEASES:
Diseases of Cervix.
Diseases of Endometrium
Diseases of Fallopian tubes
Diseases of Ovaries
Obstetric pathology
200
Bening diseases of Brest
Diseases of mail genitalia
IX. DISEASES OF ENDOCRINE SYSTEM:
Diseases of pituitary body
Diseases of adrenal glands
Diseases of Thyroid gland
Diabetes mellitus
X. PRENATAL PATHOLOGY:
Gametopaties
Blastopaties
Embryopaties
Fetopathies
XI. PERINATAL PATHOLOGY
XII. INFECTIOUS DISEASES:
Viral Diseases. AIDS.
Bacterial infections of childhood
Gastrointestinal infections
Tuberculosis
Syphilis
XIII. SEPSIS. SEPTIC SHOCK