A- Changing states and particle theory
Exercise 1: Answer the following questions by filling in the blanks.
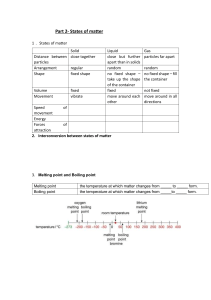
1. What are the three states of matter?
The three states of matter are _______________, _______________ and ____________
2. What are the properties of solids, liquids and gases?
Solids have _______________ volume and shape.
Liquids have ________________ volume but ____________ shape.
Gases have _______________ volume and shape.
3. What happens when we heat a solid and a liquid?
A solid will _______________ and turn into a liquid.
A liquid will _______________ and turn into a gas.
4. What happens when we cool a liquid and a gas?
A liquid will _______________ and turn into a solid.
A gas will _______________ and turn into a liquid.
5.
1
Exercise 2 :
2
1.
2.
Exercise 3:
BMelting and boiling point
3
Exercise 4:
1.
The melting point is the temperature at which a substance changes from a _solid__ to a
________
2.
The boiling point is the temperature at which a substance changes from a ________ to a
________
3.
Water melts at _______°C and boils at __________°C
4. What is the physical state of water at these temperatures?
e.g. 45 °C = liquid
125 °C = ____________________
-24 °C = ____________________
75 °C = ____________________
Exercise 5:
1Label each of the 3 pictures with the correct state of matter.
2Label each of the arrows with the name of the change in state.
Increase in temperature
Temperature at which the change of state occurs ______°C
Temperature at which the change of state occurs ______°C
4
C-Diffusion
Exercise 6:
Jena put a purple crystal into a beaker of water at 10 o’clock. She came back and looks at the beaker at 12 o’clock and 4 o’clock.
5
D- Gas pressure
Exercise 7: A student filled a balloon with air. He left it in the sun for 3 hours.
A- Explain using the diagram what happens to the particles inside the balloon.
……………………………………………………………………………………………………………..
B- Does gas pressure increase or decrease. Explain why .
…………………………………………………………………………………………..............................
........................................................................................................................................................
C- Complete:
Gas pressure …………………………………… when we compress the gas, because particles become ………………………………….. and hit the walls of the container more frequently.
Gas pressure …………………………………… when cooling, because particles ……………...energy and move ……………………………… so the collisions with the walls of the container are less.
6