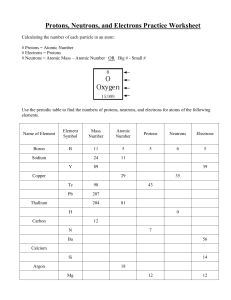
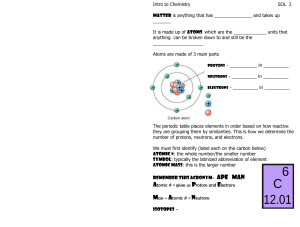
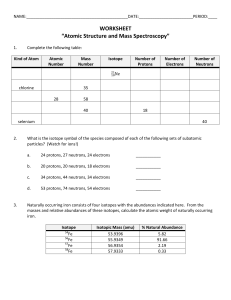
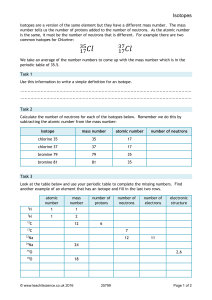
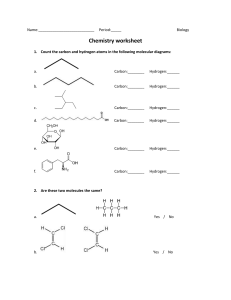
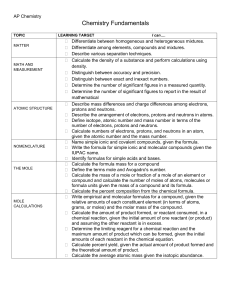
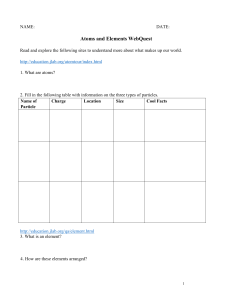
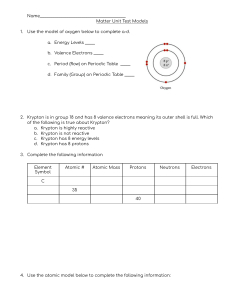
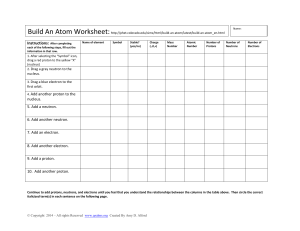
Chemistry Atoms and the Periodic Table Name_________________________ Goal: To be able to identify the number of protons, neutrons and electrons in a neutral atom from the information provided in the Periodic Table. Finish the table below. For the purpose of this exercise, round the average atomic mass on the Periodic Table to the nearest whole number to determine the mass number. Remember: number of neutrons = mass number – atomic number (number of protons) Name Symbol Atomic Number Mass Number Number of Protons Number of Neutrons Number of Electrons Calcium Hydrogen Carbon Bromine Aluminum Lithium Barium Oxygen 9 2 11 Potassium Cl 56 Chromium 92 Nitrogen 6 5 Zn 10 32 12 47 Gold 28 55



![[C3-Worksheet Reference] Atoms, Elements & Compounds](http://s3.studylib.net/store/data/025600544_1-ac81b719e1b567117977c0e1deb5ecd6-300x300.png)