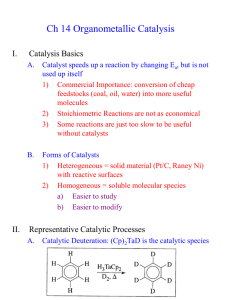
Transition metal complex possessing a formal metal to carbon double bond are termed as alkylidene or carbene complexes. There are primarily two types of metal carbenes: Fischer-type and Schrock-type carbenes. where X, Y = alkyl, aryl, H, or heteroatom (O, N, S, Halogen) Schrock carbene: Schrock carbene is nucleophilic and is named after the dicoverer Richard R. Schrock. Characteristics: sp hybridized and linear (triplet) Carbon atom in carbene is nucleophilic (Electron rich) Carbene-attacked by electrophiles High oxidation state early transition metals such as Ti(IV) and Ta(V) Substituents are Hydrogen, alkyl, cp, Cl etc. Ligands are generally good σ or π donors e.g., Bonding in such complexes can be viewed as the coupling of a triplet state metal and triplet carbine. Popular Schrock carbenes used in Olefin Metathesis: Preparation: The Petasis reagent is prepared by the salt reaction of methylmagnesium chloride or with titanocene dichloride. Cp2TiCl2 + 2 CH3MgCl → Cp2Ti(CH3)2 + 2 MgCl2 The Tebbe’s reagent is synthesized from diemethyltitanocene as follows, Cp2Ti(CH3)2 + Al(CH3)2Cl → Cp2TiCH2 AlCl(CH3 )2 + CH4 Olefin Metathesis reaction: General: Olefin Metathesis reactions: