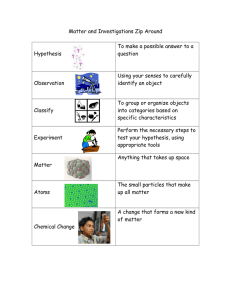
Caroline gitau Chem 1405-33432 Vocabulary 1 1. 1.chemistry-the science which deals with composition and properties of substances and various elementary forms of matter . 1. 2. matter.is a substance that has inertia and occupies physical space 2. 3. three different levels of matter –liquid, solid and gases 3. 1. 4. model –a synthetic coordination entity that closely approaches the properties of a metal ion in protein and yields useful information concerning biological structure and function. 1. 5. ball-and-stick model – are three-dimensional models where atoms are represented by spheres of different colors and bonds are represented by sticks between the spheres. 2. 1. 6. space-filling model –are similar to ball and stick models in that they are three-dimensional models that represent atoms as colored spheres 2. 1. 7. symbol - is a notation of one or two letters representing a chemical element. 1. 8.State of matter One of the four principal conditions in which matter exists-solid, liquid, gas, and pl asma. Seealso phase phase transition . 1. 9. kinetic molecular theory – A theory of the thermodynamic behavior of matter, especially the relationships among pressure, volume, and temperature in gases, based on the dependence of temperature on the kinetic energy of the rapidly moving particles of a substance. 1. 10.gas – is defined as a state of matter consisting of particles that have neither a defined volume nor defined shape 1. 11.liquid -is one of the states of matter 1. 12.solid – is a state of matter characterized by particles arranged such that their shape and volume are relatively stable 1. 13.physical properties . is a characteristic of matter that may be observed and measured without changing the chemical identity of a sample 1. 14. physical changes – Any change that occurs without altering the chemical composition of a substance 2. 15. chemical change – Chemical changes occur when a substance combines with another to form a new substance 16. Evidence of a chemical change -The following can indicate that a chemical change has taken place, although this evidence is not conclusive: Change of odor. Change of color (for example, silver to reddish-brown when iron rusts). 1. 17.homogeneous – mixture is a solid, liquid, or gaseous mixture that has the same proportions of its components throughout any given sample 1. 18. solution – s a special type of homogeneous mixture composed of two or more substances. 1. 19. heterogeneous – This is one which has a non-uniform composition. Example A mixture of sand and water 1. 20. pure substance -is a material that has a constant composition (is homogeneous) and has consistent properties throughout the sample. 21. mixture – Any substance that has a uniform and unchanging composition is considered to be pure 1. 22. element – is a substance whose atoms all have the same number of protons: 1. 23. compound – is a substance formed when two or more chemical elements are chemically bonded together. 24. elemental symbol – is a notation of one or two letters representing a chemical element. 1. 25. chemical formula – tells us the number of atoms of each element in a compound. 26. atom -is the smallest component of an element. 1. 27. Law of Definite Composition – states that agiven chemical compound always contains its component elements in fixed ratio (by mass) and does not depend on its source and method of preparation. 28. force field – special case of energy functions or interatomic potentials; 1. 29.static electricity –is often created when two objects that are not good electrical conductors are rubbed together, and electrons from one of the objects rub off onto the other 1. 30.chemical equation – is the symbolic representation of a chemical reaction in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side 1. 31. reactants - are the starting materials in a chemical reaction 1. 32. products – are the species formed from chemical reactions 2. 33. exothermic reaction – is a chemical reaction that releases energy through light or heat. 34. endothermic reaction – process is any process which requires or absorbs energy from its surroundings ... 35. potential energy – the energy possessed by a body by virtue of its position relative to others, stresses within itself, electric charge, and other factors. 1. 36. kinetic energy – energy which a body possesses by virtue of being in motion. 1. 37. Law of Conservation of Mass – is neither created nor destroyed in chemical reactions. In other words, the mass of any one element at the beginning of a reaction will equal the mass of that element at the end of the reaction. 1. 38. Law of Conservation of Energy – is one of the basic laws of physics and therefore governs the microscopic motion of individual atoms in a chemical reaction.