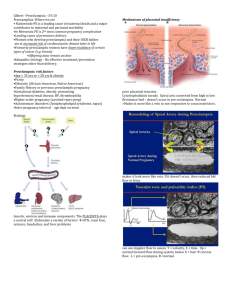
Understanding my patient The patient is a 31 year old G3P1011 at 12 weeks and 5 days gestation presenting with headaches and blurry vision. She has a history of severe preeclampsia with her prior pregnancy requiring induction and “cervical insufficiency” requiring cerclage at 17 weeks for dilation and vaginal bleeding- this pregnancy was carried to term and she had a C-section with no complications. She presents to clinic for a follow up after an ED visit 4 days ago. At that time she reports a bilateral frontal headache described as a “tightness” with associated phonophobia and photophobia and blurry vision. She states she kept removing her glasses to clean them but then noticed they weren’t dirty. She checked her BP at home multiple times, at least 4 hours apart, and the readings were 138/94, 140/97, 140/98. She also reports nausea today, but denies vomiting, leg swelling, no RUQ abdominal pain. She states she has a history of migraines but only during her last pregnancy. In the year since she delivered her son, she reports her BP has been normal and she has not had headaches. She denies vaginal bleeding. Cervical insufficiency and cerclage? Cervical insufficiency is the inability of the uterine cervix to retain a pregnancy in the 2nd trimester in the absence of contractions, labor, or both. It is recommended to place “history based cerclage” at 12-14 weeks gestation in women with prior cervical insufficiency. Although there is not sufficient data to support it, many practitioners also start hydroxyprogesterone caproate weekly at 16 weeks until 36 weeks. As cervical length screening after cerclage placement would not change management, it is not recommended. Our patient would have had a “rescue or physical exam based cerclage” based on dilation without contractions placed at 17 weeks during her prior pregnancy. Systematic reviews show that cerclage in this instance improves pregnancy outcome (neonatal survival 71 percent with cerclage versus 43 percent with expectant management; relative risk 1.65, 95% CI 1.19-2.28). Cerclage after 24 weeks may cause accidental rupture of the fetal membranes leading to early preterm birth. There may be increased risk of chorioamnionitis with cerclage. The third type, “USS indicated cerclage”, is performed when exam shows cervical shortening </= to 2.5 cm in women with history of cervical surgery or preterm birth. However the efficacy of USS indicated cerclage compared to conservative management is controversial- a recent study showed that women whose cerclage is placed distally or whose cervix fails to lengthen after cerclage are at increased risk of preterm birth. Hypertension in pregnancy Chronic hypertension? Hypertension present before pregnancy or before the 20th week, or persists longer than 12 weeks after. Approx. ⅓ will develop superimposed preeclampsia! Gestational hypertension? Hypertension that develops after the 20th week of gestation in the absence of proteinuria. Approx 50% develop preeclampsia, 10% develop eclampsia! Preeclampsia? Severe features? HELLP? Superimposed preeclampsia? 24 hour urine >300 mg protein. In patients who have baseline renal disease, elevated uric acid above 6 is used to differentiate preeclampsia from exacerbation of hypertension. If any severe signs are present, this is a diagnosis of severe superimposed preeclampsia. Eclampsia? Tonic clonic seizure, usually during or around delivery, usually in patient with preeclampsia. Patient may not have known preeclampsia prior to presentation with seizure. Which of the following is NOT a risk factor for preeclampsia? Multiple gestation in utero Chronic HTN New paternity Chronic renal disease Smoking Collagen vascular disease, like SLE Paternal grandmother of fetus had preeclampsia Pregestational diabetes Cohabitating with father of baby < 1 year African American race Previous preeclampsia Maternal age <20 or >35 Living at high altitudes Nulliparity Fetus with Trisomy 13 Use of condoms with partner before conception Abnormal remodeling of spiral arteries — In normal pregnancies, the cytotrophoblast cells of the developing placenta migrate through the decidua and part of the myometrium to invade both the endothelium and highly muscular tunica media of the maternal spiral arteries, the terminal branches of the uterine artery that supply blood to the developing fetus/placenta (figure 1). As a result, these vessels undergo transformation from small muscular arterioles to large capacitance vessels of low resistance, thus greatly facilitating blood flow to the placenta compared with other areas of the uterus [7,8]. Remodeling of the spiral arteries probably begins in the late first trimester and is completed by 18 to 20 weeks of gestation, although the exact gestational age at which trophoblast invasion of these arteries ceases is unclear. By comparison, in preeclampsia, cytotrophoblast cells infiltrate the decidual portion of the spiral arteries, but fail to penetrate the myometrial segment [9,10]. The spiral arteries fail to develop into large, tortuous vascular channels created by replacement of the musculoelastic wall with fibrinoid material; instead, the vessels remain narrow, resulting in placental hypoperfusion (figure 2 and figure 3). This defect in deep placentation has been associated with development of multiple adverse pregnancy outcomes, including second trimester fetal death, abruptio placentae, preeclampsia with or without intrauterine growth restriction, intrauterine growth restriction without maternal hypertension, premature Immunologic Theory -Immunologic intolerance between the mother and fetus may play a role in the pathogenesis of preeclampsia. -Abnormalities similar to those observed in organ rejection graft versus host disease, have been observed in preeclamptic women. - Interaction between NK cells and EVT cells has been hypothesized to control placental implantation. The extravillous trophoblast (EVT) cells express HLA class I antigens, the NK cells express a variety of receptors . They infiltrate the maternal decidua in close contact with the EVT cells. In this way the NK cells should, in a healthy placenta, stimulate vascular invasion by EVT cells. - In preeclampsia, conflict between maternal and paternal genes is believed to induce abnormal placental implantation through increased (aberrant) NK cell activity. - Placental bed biopsies from women with preeclampsia have revealed increased dendritic cell infiltration in preeclamptic decidual tissue, which initiate antigen-specific T-cell responses to transplantation antigens. Expectant management? Daily labs including CBC, liver enzymes, BMP, uric acid Give betamethasone! Daily fetal US, NST Antihypertensives - labetalol or nifedipine ?Seizure prophylaxis What about prevention? Low dose aspirin has been shown to have beneficial effects on the prevention of the development of preeclampsia. What is the mechanism? This paper discovered that LDA causes a reduction of sFlt-1 release, an antiangiogenic tyrosine kinase that antagonizes VEGF and PIGF (placental growth factor) and causes endothelial dysfunction. This is in addition to the reduction of thromboxane A2, which is also a factor produced in preeclampsia that favors systemic vasoconstriction. LDA affects COX1 more than COX2, which selectively inhibits TXA1 without affecting prostacyclin levels, favoring systemic vasodilation. Other findings: LDA had protective effect on cell apoptosis triggered by hypoxia. It caused improved cell migration activity and invasion of trophoblast cells as well as ability to form tubes.