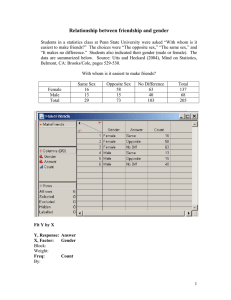
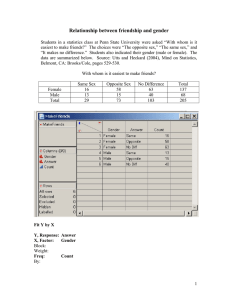
Organic Chemistry, 7e (Wade) Chapter 14 Ethers, Epoxides, and Sulfides 1) What is the hybridization of the oxygen atom in dialkyl ethers? A) sp3 B) sp2 C) sp D) s E) p Answer: A Diff: 1 Section: 14.2 2) Which of the following corresponds to the COC bond angle in dimethyl ether? A) 60° B) 94° C) 110° D) 122° E) 180° Answer: C Diff: 1 Section: 14.2 4) Draw structures which show the hydrogen bonding interaction that exists between water and dimethyl ether. Answer: Diff: 2 Section: 14.2 7) What complex results when BF3 is dissolved in dimethyl ether? Answer: (CH3)2O∙BF3 Diff: 2 Section: 14.2 8) What is the correct IUPAC name for the following compound? A) 3-methyl-3,4-epoxypentan-1-ol B) 5-hydroxy-3-methyl-2,3-epoxypentane 1 C) 1-(2-hydroxyethyl)-1,2-dimethyloxirane D) 3,4-epoxy-3-methylpentan-1-ol Answer: A Diff: 2 Section: 14.3 9) Which of the following is not a true statement? A) Dioxanes are six-membered ring ethers. B) Furans are five-membered ring ethers. C) Oxiranes are three-membered ring ethers. D) Oxetanes are five-membered ring ethers. E) Pyrans are six-membered-ring ethers. Answer: D Diff: 2 Section: 14.3 10) What is the complete systematic IUPAC name for the following compound? A) 4-(1-methylethoxy)-4-isopropyl-4-methylpent-2-ene B) 4-isopropyl-2,4-dimethylhept-5-en-3-ol C) isopropyl-(4-isopropyl-4-methylbut-2-enyl) ether D) 4-isopropoxy-4,5-dimethylhex-2-ene E) 4-isopropyl-4-methylbut-2-en-isopropyl ether Answer: D Diff: 2 Section: 14.3 14) Provide the IUPAC name of the compound shown below. Answer: 1,1-diethoxycyclohexane Diff: 2 Section: 14.3 2 15) Provide a structural representation of 2-ethoxypentane. Answer: Diff: 1 Section: 14.3 16) Provide a structural representation of isopropyl tert-butyl ether. Answer: (CH3)2CHOC(CH3)3 Diff: 1 Section: 14.3 17) Provide a structural representation of 1,2-epoxybutane (also called 2-ethyloxirane). Answer: Diff: 1 Section: 14.3 20) Provide a structural representation of cis-3-ethyl-1,2-epoxycyclopentane. Answer: Diff: 2 Section: 14.3 24) Propose a structure for the ether of formula C4H10O with the following 1H NMR signals: δ 1.20 (triplet, 6H), δ 3.45 (quartet, 4H) (ppm). Answer: CH3CH2OCH2CH3 Diff: 2 Section: 14.4 25) Propose a structure for the ether of formula C4H10O with the following 1H NMR signals: δ 0.95 (triplet, 3H), 1.52 (multiplet, 2H), 3.30 (singlet, 3H), 3.40 (triplet, 2H) (ppm). Answer: CH3CH2CH2OCH3 Diff: 2 Section: 14.4 3 26) Propose a structure for the ether of formula C4H10O with the following 1H NMR signals: δ 1.13 (doublet, 6H), 3.30 (singlet, 3H), 3.65 (septet, 1H) (ppm). Answer: (CH3)2CHOCH3 Diff: 2 Section: 14.4 27) Provide an acceptable name for the compound shown below. CH3OCH2CH(CH3)2 Answer: isobutyl methyl ether or 1-methoxy-2-methylpropane Diff: 2 Section: 14.4 28) Provide an acceptable name for the compound shown below. Answer: trans-2-ethoxycyclohexan-1-ol Diff: 2 Section: 14.4 29) Provide an acceptable name for the compound shown below. Answer: propylene oxide or methyloxirane Diff: 2 Section: 14.4 30) What is the chemical shift of the carbon bound to oxygen in ethers in 13C NMR? Answer: δ 65 to δ 90 Diff: 3 Section: 14.4 31) Which pair of reagents would produce the highest yield of (R)-2-ethoxybutane? A) sodium (S)-2-butoxide + iodoethane B) sodium (R)-2-butoxide + iodoethane C) sodium ethoxide + (S)-2-iodobutane D) sodium ethoxide + (R)-2-iodobutane E) Both B and C would work equally well. Answer: B Diff: 2 Section: 14.5 4 32) Which of the following is an acceptable way to synthesize t-butyl ethyl ether? A) treatment of t-butyl bromide with sodium ethoxide B) treatment of ethyl bromide with sodium t-butoxide C) heating a mixture of ethanol and t-butanol in sulfuric acid D) treating t-butyl bromide with Hg(OAc)2 E) treating t-butanolwith Hg(OAc)2 Answer: C Diff: 2 Section: 14.5 33) Which of the following reactions is classified as a Williamson ether synthesis? A) B) C) D) E) Answer: B Diff: 2 Section: 14.5 34) The Williamson ether synthesis occurs by the __________ mechanistic pathway. Answer: SN2 Diff: 1 Section: 14.5 5 35) Show the best method for preparing methoxycyclopentane via the Williamson ether synthesis. Answer: Diff: 1 Section: 14.5 36) Provide the major organic product in the reaction below. CH3CH2CH2OH → Answer: CH3CH2CH2OCH2Ph Diff: 1 Section: 14.5 37) Show the best method for preparing 4-propoxytoluene via the Williamson ether synthesis. Answer: Diff: 2 Section: 14.5 38) Provide the sequence of reactions by which propoxycyclohexane can be prepared through a Williamson ether synthesis. Answer: Diff: 2 Section: 14.5 6 39) Provide the major organic product of the following reactions. Answer: Diff: 2 Section: 14.5 40) Provide the major organic product of the reaction shown below. Answer: Diff: 2 Section: 14.5 41) Provide the major organic product of the reaction shown below. Answer: 7 Diff: 2 Section: 14.5 42) Provide the necessary reagents to complete the following reaction. Answer: Diff: 3 Section: 14.5 43) Provide the major organic product in the reaction below. Answer: Diff: 1 Section: 14.5 44) When cyclohexene is subjected to mercuration in methanol and the resulting mixture is reduced with sodium borohydride, the major organic product is: A) a meso ether B) a 1:1 mixture of enantiomeric ethers C) a meso diol D) a 1:1 mixture of enantiomeric diols E) methoxycyclohexane Answer: E Diff: 2 Section: 14.6 8 45) When pent-1-ene is treated with mercury(II) acetate in methanol and the resulting product is reacted with NaBH4, what is the primary organic compound which results? A) 3-ethoxypentane B) 1-methoxypentane C) 1-ethoxypentane D) 2-ethoxypentane E) 2-methoxypentane Answer: E Diff: 2 Section: 14.6 46) Which of the following reactions or series of reactions will lead to the formation of methoxycyclohexane? A) B) C) D) E) Answer: D Diff: 2 Section: 14.6 47) Provide the major organic product of the following reactions. 9 Answer: Diff: 2 Section: 14.6 48) Provide the major organic product of the following reactions. Answer: Diff: 2 Section: 14.6 49) Show how propyl cyclopentyl ether can be prepared starting with cyclopentene and using an alkoxymercuration-demercuration route. Answer: Diff: 2 Section: 14.6 50) Show the reagents necessary to prepare 1-ethoxy-1-methylcyclopentane from 1methylcyclopentene. Answer: 1. Hg(OAc)2, CH3CH2OH 2. NaBH4 Diff: 1 Section: 14.6 51) Provide the major organic product in the reaction below. CH3CH2CH=CH2 1. Hg(OAc)2 ,CH3CH2OH → 2. NaBH4 10 Answer: CH3CH2CHCH3 ∣ OCH2CH3 Diff: 2 Section: 14.6 52) Show the reagents necessary for the conversion of 1-bromo-1-methylcyclopentane to 1ethoxy-1-methylcyclopentane. Answer: CH3CH2OH, heat Diff: 2 Section: 14.6 53) When hexan-1-ol is treated with conc. H2SO4 at moderate temperatures, __________ is formed via a(n) __________ mechanism. A) di-n-hexyl ether, SN2 B) di-n-hexyl ether, SN1 C) di-n-hexyl ether, E2 D) di-n-propyl ether, E1 E) hex-1-ene, SN1 Answer: A Diff: 2 Section: 14.7 54) Some ethers can be prepared by heating the appropriate alcohol in the presence of sulfuric acid. Can di-sec-butyl ether be prepared from sec-butanol by this route? Explain your answer. Answer: No. sec-Butanol is a secondary alcohol. Secondary and tertiary alcohols predominatly undero elimination when heated in acid. Diff: 2 Section: 14.7 11 55) Suggest a reasonable mechanism for the reaction shown below. Answer: or Diff: 2 Section: 14.7 56) Provide two reasons why it would be difficult to prepare ethoxycyclopentane via an intermolecular dehydration route from ethanol and cyclopentanol. Answer: 1. Secondary alcohols tend to yield alkenes under these conditions. 2. Symmetrical ethers would be produced as well. Diff: 2 Section: 14.7 57) Provide the major organic product in the reaction below. Answer: Diff: 2 Section: 14.7 12 58) Can one prepare di-sec-butyl ether in good yield by heating butan-2-ol in the presence of sulfuric acid? Explain. Answer: This would not be an appropriate method of preparing di-sec-butyl ether in good yield. When butan-2-ol, a 2° alcohol, is heated in the presence of strong acid, the major product is 2butene, the elimination product. Diff: 2 Section: 14.7 59) Di-n-pentyl ether can be converted to 1-bromopentane by treatment with HBr through essentially a(n) __________ mechanism. A) SN2 B) SN1 C) E2 D) E1 E) ring opening Answer: A Diff: 1 Section: 14.8 60) What are the expected products of the reaction of PhOCH3 with concentrated HI? A) phenol and methanol B) phenol and iodomethane C) iodobenzene and methanol D) iodobenzene and iodomethane Answer: B Diff: 1 Section: 14.8 61) Treatment of tetrahydrofuran with excess HBr results in the formation of what major organic product? A) 1-bromobutane B) 1,2-dibromobutane C) 1,4-dibromobutane D) 1-bromopentane E) 1,5-dibromopentane Answer: C Diff: 2 Section: 14.8 13 62) What is the major organic product which results when tetrahydrofuran is reacted with excess HBr? A) 1,2-dibromobutane B) 1,3-dibromobutane C) 1,4-dibromobutane D) 4-bromobutan-1-ol E) 3-bromobutan-1-ol Answer: C Diff: 3 Section: 14.8 63) Provide the major organic product of the following reactions. Answer: Diff: 2 Section: 14.8 64) Provide the major organic product of the reaction shown below. Answer: Diff: 2 Section: 14.8 14 65) Provide the major organic product of the reaction shown below. Answer: Br(CH2)6Br Diff: 2 Section: 14.8 66) Provide the major organic product(s) in the reaction below. Answer: Diff: 1 Section: 14.8 67) Predict the products of the following reaction and give a reasonable mechanism for their formation. Answer: Diff: 2 Section: 14.8 15 68) Provide the major organic product in the reaction below. Answer: Diff: 3 Section: 14.8 71) Which of the following is produced by the reaction of (CH3CH2)2S with CH3CH2I? A) CH3CH2CH2CH2I B) (CH3CH2)3S+ IC) (CH3CH2)3S D) CH3SCH2CH2CH3 E) CH3CH2SCH2CH2I Answer: B Diff: 2 Section: 14.10 73) What term is given to the sulfur analogues of ethers? Answer: sulfides or thioethers Diff: 1 Section: 14.10 74) Provide a common name for the following compound. Answer: allyl ethyl sulfide Diff: 1 Section: 14.10 75) Provide the major organic product of the following reactions. Answer: Diff: 2 Section: 14.10 16 76) Provide the major organic product of the reaction shown below. Answer: Diff: 2 Section: 14.10 77) Provide the structure of the sulfonium salt that results when (CH3CH2)2S reacts with CH3CH2Cl. Through what mechanistic path is this reaction occurring? Answer: (CH3CH2)3S+ Cl-; SN2 Diff: 2 Section: 14.10 78) Why are sulfonium salts good alkylating agents? Answer: Sulfonium salts are good alkylating agents because the leaving group is an uncharged sulfide. Diff: 2 Section: 14.10 79) When trans-hex-3-ene is treated with PhCO3H, the major organic product is: A) a meso epoxide B) a 1:1 mixture of enantiomeric epoxides C) a meso diol D) a 1:1 mixture of enantiomeric diols E) hexan-3-ol Answer: B Diff: 2 Section: 14.11 80) Through what mechanims can a 1,2-halohydrin be converted into an epoxide? A) SN1 B) SN2 C) E2 D) electrophilic addition E) polymerization Answer: B Diff: 2 Section: 14.11 81) Magnesium monoperoxyphthalate is often used as a reagent in what process? 17 A) reductive amination B) dehydration C) halohydrin formation D) epoxidation E) Williamson ether synthesis Answer: D Diff: 2 Section: 14.11 82) Provide the major organic product of the following reactions. Answer: Diff: 2 Section: 14.11 83) Provide the major organic product in the reaction below. Answer: Diff: 1 Section: 14.11 84) Provide the major organic product in the reaction below. Answer: Diff: 1 Section: 14.11 18 85) Show the reagents necessary to prepare trans-1,2-cyclopentane-1,2-diol from cyclopentene. Answer: 1. PhCO3H 2. H3O+ Diff: 1 Section: 14.11 86) Suggest a reasonable mechanism for the reaction shown below. Answer: Diff: 2 Section: 14.11 87) Provide the major organic product in the reaction below. Answer: Diff: 2 Section: 14.11 88) Show the reagents necessary to prepare 1,2-epoxy-1-methylcyclopentane from cyclopentanone. Answer: 1. CH3MgBr 2. conc. H2SO4 3. PhCO3H Diff: 2 Section: 14.11 89) Provide the major organic product in the reaction below. 19 HOCH2(CH2)2CH2Br Answer: Diff: 3 Section: 14.11 90) Which of the following reactions or series of reactions will lead to formation of trans-2methoxycyclohexanol? A) B) C) D) E) Answer: D Diff: 2 Section: 14.12 91) What results when but-1-ene is subjected to the following reaction sequence: (1) Cl2, H2O, (2) NaOH, (3) H3O+? A) a meso epoxide B) a 1:1 mixture of enantiomeric epoxides C) a meso diol D) a 1:1 mixture of enantiomeric diols E) butan-2-ol Answer: D Diff: 3 20 Section: 14.12 92) Use arrows to show correct electron flow in the mechanism for the reaction below. Include all intermediate structures correctly drawn with formal charges. Answer: Diff: 3 Section: 14.12 93) What is the stereochemistry of the product of the acid hydrolysis of trans-2,3-epoxybutane? Answer: meso Diff: 2 Section: 14.12 94) What results when cyclopentene oxide is treated with aqueous base? A) a racemic mixture of trans-1,2-diols B) a meso trans-1,2-diol C) a racemic mixture of cis-1,2-diols D) a meso cis-1,2-diol E) cyclopentene Answer: A Diff: 2 21 Section: 14.13 95) What reagents are necessary for the following transformation? Answer: Diff: 3 Section: 14.13 96) Provide a structure for the expected product of the following reaction. Answer: Diff: 3 Section: 14.13 97) Provide the major organic product in the reaction below. Answer: Diff: 3 22 Section: 14.14 98) Provide the major organic product in the reaction below. Answer: Diff: 2 Section: 14.14 99) Provide the major organic product in the reaction below. Answer: Diff: 2 Section: 14.14 100) Provide the major organic product in the reaction below. Answer: Diff: 2 Section: 14.14 101) Provide the major organic product in the reaction below. Answer: 23 Diff: 3 Section: 14.14 102) Show the reagents necessary to prepare trans-2-deuteriocyclohexan-1-ol from cyclohexene. Answer: 1. PhCO3H 2. LiAlD4 Diff: 3 Section: 14.14 103) Show the reagents necessary to prepare the compound below from cyclohexanone. Answer: 1. CH3MgBr 2. conc. H2SO4 3. PhCO3H 4. CH3CH2ODiff: 3 Section: 14.14 104) What is the major difference in the structure of the product formed in a Grignard reaction when an alkyl magnesium halide (Grignard reagent) is reacted with an epoxide rather than an aldehyde or ketone followed by work-up with H3O+? A) The alkyl group from the Grignard reagent will be bonded to the α-carbon of the alcohol product. B) The alkyl group from the Grignard reagent will be bonded to the β-carbon of the alcohol product. C) The alkyl group from the Grignard reagent will form an ether product rather than an alcohol product. D) Two equivalents of the Grignard will result in the bonding of two alkyl groups to the αcarbon of the alcohol product. E) The epoxide will protonate the Grignard reagent resulting an alkane product. Answer: B Diff: 1 Section: 14.15 24 105) What compound is formed when ethylene oxide is reacted with n-pentyllithium followed by treatment with aqueous acid? A) 1-heptanol B) 2-heptanol C) heptanal D) 2-heptanone E) pentanal Answer: A Diff: 2 Section: 14.15 106) What compound is formed when methyloxirane is reacted with ethylmagnesium bromide followed by treatment with aqueous acid? A) 1-pentanol B) 2-pentanol C) 2-methyl-1-butanol D) 2-methyl-2-butanol E) 3-methyl-1-butanol Answer: B Diff: 2 Section: 14.15 107) What compound is formed when 2,2-dimethyloxirane is treated with ethanol containing a trace of HCl? A) 2-ethoxy-2-methyl-1-propanol B) 1-ethoxy-2-methyl-2-propanol C) 2-ethoxy-2-methyl-2-propanol D) 2-ethoxy-1-butanol E) 1-ethoxy-2-butanol Answer: A Diff: 2 Section: 14.15 108) Provide the major organic product in the reaction below. Answer: Diff: 2 Section: 14.15 25 109) Provide the major organic product of the following reactions. Answer: Diff: 2 Section: 14.15 110) Provide the major organic product in the reaction below. Answer: Diff: 2 Section: 14.15 111) Show the reagents necessary to prepare 2-phenylethanol from bromobenzene. Answer: 1. Mg, ether 2. oxirane 3. H3O+ Diff: 2 Section: 14.15 26