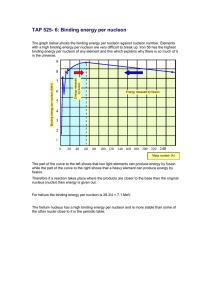
Name: ________________________________ IB Physics Topic 7.2 ACTIVITY: Nuclear Binding Energy Purpose: To understand how the total nuclear binding energy and the binding energy per nucleon vary from nuclide to nuclide. In this activity, you are going to use an internet database of properties of the elements in the periodic table in order to plot two graphs of nuclear properties and then use these to see some trends. You will do this by copying the information from the internet database into a spreadsheet program (Excel) and then using the spreadsheet’s Chart Wizard to make your graphs. 1. First, open the spreadsheet program Excel. You should see a blank grid of many empty boxes (cells) into which you are going to paste data. Notice that each cell is identified by a vertical column letter (A,B,C…) and a horizontal row number (1,2,3…). Thus, the first cell in the upper left hand corner is named A1 and to the right of it is A2 while below it is B1, etc. 2. The data in the internet database will come in the following form: Notice that there are 10 columns of information to import into the spreadsheet although we will only use two of them: nucleon number (mass number = A) and Binding Energy (BE). Unfortunately, there is no way to copy just these two columns so we will have to copy them all and delete what we don’t want later. 3. In Excel, label column 2 “Nucleon Number” (in cell B1) and label column 5 “Binding Energy” (in cell E1). Now you’re ready to import the data. 4. Go to http://hyperphysics.phy-astr.gsu.edu/hbase/pertab/pertab.html#c1 to see the Periodic Table of Elements. Each element is clickable and will give you both atomic and nuclear data. Start off by choosing Hydrogen and scrolling down to the Nuclear Data chart. Highlight all three rows (and ten columns) of nuclear data and right-click to copy this data. 5. Now go back to Excel to paste in this data. Do this by right clicking in cell A2 and clicking Paste. You should now have all the nuclear data about hydrogen in your spreadsheet. 6. One good thing about spreadsheets is that it’s easy to put lots of data in them and that’s what we need to do. Repeat what you did for hydrogen for each of the first ten elements of the periodic table, then for every fifth element thereafter. Also, include each of the elements 24-28 as well as Uranium and Plutonium. Try to do this in numerical order so that it’s easier to format the graph later on. That’s a lot of data! (Good thing you didn’t have to enter it by hand!) 7. We’re almost ready to start processing the data and graphing it. First though, it’s a good idea to save this data file in case you make a mistake. Save the file on the desktop or your flash drive. 8. Now, you should get rid of the data columns you don’t need. You will only need the columns for Nucleon Number and Binding Energy so you can delete the others. Do this by highlighting the letter at the top of each column and right clicking Delete. When you’re done, you should have Nucleon Number in column A and Binding Energy in column B. You’re now ready to graph and analyze. Graph #1: Binding energy vs. nucleon number 9. Click on Insert/Chart to get the Chart Wizard to help you make a graph. Choose an XY(Scatter) chart and choose “Scatter with data points connected by smoothed lines.” 10. Choose the Series tab and click Add. To add your X-values, click on the grid icon to the right of X values. The chart wizard will minimize to enable you to select what you want to graph on the x-axis. Highlight (click and drag) over the values in the Nucleon Number column then hit Enter. (Don’t highlight the title cell.) 11. To add your Y-values, click on the grid icon to the right of Y values (but first clear out any stray symbols that may be in the box). The chart wizard will minimize to enable you to select what you want to graph on the y-axis. Highlight (click and drag) over the values in the Binding Energy column then hit Enter. At this point you should see your data graphed. 12. Click Next so you can format the graph. Click on the Titles tab and enter a Chart title and titles (Values) for the x and y axes. Click Next and choose to place the chart as New Sheet then click Finish. This will expand the chart to a full page for printing. 13. However, in order to save ink, before printing you should make sure that the chart background is white instead of grey. To do this, double click on the chart’s background to bring up the Format Plot Area box. For area, choose None and then click OK. 14. Print your chart. Graph #2: Binding energy per nucleon vs. nucleon number Now you are going to make a chart for the binding energy per nucleon versus the nucleon number for each nuclide in your data table. 15. You’ll first have to add a new column of processed data (column C) for the binding energy per nucleon. To do this, you’ll have to divide the Binding Energy column by the Nucleon Number column. First, click on the top cell of column C and label it “BE per nucleon.” 16. Then, click on the next cell down (C2). To write a formula to process data in Excel, you must first type =. Then, you must type in your formula using the names of the cells. So to divide the binding energy (in cell B2) by the nucleon number (in cell A2), you must type in B2/A2. Altogether, your formula will be =B2/A2. 17. Then, copy and paste this cell to all the other cells in column C to duplicate the formula. To do this, right click on the cell and choose Copy, then click on the next cell below (row 3) and drag all the way down the column to highlight it. Then, right click in the highlighted area and choose Paste. You should now see numbers filling column C. These are the numbers you are going to graph next. 18. To graph the binding energy per nucleon vs. nucleon number, follow the same steps as you did for the first graph, except choose to graph column C on the y-axis. Prepare your graph and print it out. Conclusion Questions: Inspect your graphs and your data table to answer the following questions. 1. As the nucleon number increases, what happens to the total binding energy of a nucleus? [2] 2. As the nucleon number increase, what happens to the binding energy per nucleon of a nucleus? [3] 3. Which nuclide has the highest binding energy per nucleon? [2] 4. The majority of the binding energy per nucleon values are between what two values? (Give[2]your answers to the nearest whole numbers of MeV.) 5. What is the atomic number for iron? 6. What is the atomic number for uranium? [1] [1] [2] 7. As you move from hydrogen to iron on the periodic table, what happens to the binding energy per nucleon? 8. As you move from iron to uranium on the periodic table, what happens to the binding energy [2] per nucleon? [40] 9. Be sure to attach both graphs to this lab.