Percentage composition and empirical formula pac teacher notes

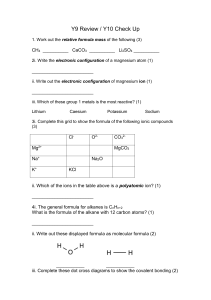
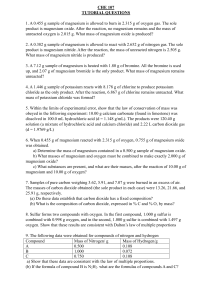
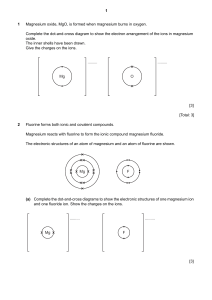
Heinemann Chemistry 1 5e
Chapter 5 Quantifying atoms and molecules
Teacher
Notes
Section 5.4
Percentage composition and empirical formulas
Experiment 5.4.1
Chemical composition of a compound
Rationale
This experiment develops students’ skills in quantitative chemistry, and acts as an introduction to empirical formula determina tion. In general, and allowing for error, students’ results should support the idea of constant chemical composition.
Background knowledge
Students should know that when magnesium is heated in air, magnesium oxide is formed.
Materials
Per group of 20 students working in pairs:
• 10 lengths of magnesium ribbon, each 20–40 cm long (assign a different length to each pair of students)
• 10 steel wool pieces
• 10 crucibles and lids
• 10 pipeclay triangles
• 10 Bunsen burners
• 10 bench mats
• 10 tripods
• 10 tongs
• 10 electronic balances
• 20 pairs of safety glasses
Safety
• Warn students against looking at burning magnesium.
Hints
This experiment is subject to considerable errors. The actual percentage of magnesium in magnesium oxide is 60%. How ever, results ranging from 55% to 70% are usual.
Answers
3 424 tonnes
4 TeO
2
5 Percentage composition refers to a mass ratio (or percentage), whereas empirical formula gives a mole ratio of elements present.
Extension activity
If students are familiar with the mole concept, the coefficients in the equation
xMg(s) + yO
2
(g) → zMgO(s) can be determined.
Copyright © Pearson Australia 2016 (a division of Pearson Australia Group Pty Ltd) ISBN 978 1 4886 1124 7 Page 1