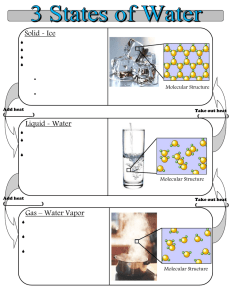
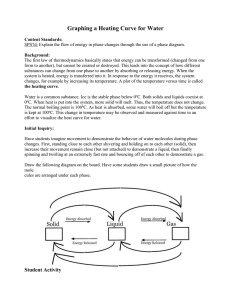
Name _____________________________________________Date___________________________ Period _____ States of Matter Lab Objective: To determine how water behaves when its temperature is increased Hypothesis: Write an “If….then” statement about how temperature will affect an object’s state of matter. ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ Background Information: Water exists in three states: solid, liquid, and gas. Solid water is ice. The water molecules in ice vibrate but cannot change position relative to other water molecules. In liquid water, molecules are free to move around each other. However, the molecules remain close together. Gaseous water is called water vapor. Water molecules in vapor have so much energy that they escape the attractions of the other water molecules. Water vapor spreads out into the available space. In this activity, you will investigate the three states of water. Materials: 500-mL beaker crushed ice hot plate Celsius thermometer Stop watch Safety: The hot plate and beaker will be very hot. Do not touch them until after they have cooled. Procedure: 1. Place the ice directly into the beaker. 2. Place the thermometer in ice and record the temperature in degrees Celsius in the data table. 3. Turn the hot plate on. 4. Carefully place the beaker on the hot plate and start the stop watch. 5. Record the temperature of the ice every minute. 6. Record the state of matter of the water at each minute. 7. Make a graph showing how the water temperature changed over time. a. Label the x-axis of your graph Time (min) b. Label the y-axis of your graph Temperature (Celsius) Time Temperature (C) State of Matter/Observations Analysis: 1. Examine the graph you created. Describe how the temperature changed throughout the experiment in relation to the state of matter of the water. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 2. Heat energy is being continually added to the system by the hot plate. This causes the water molecules to change their motion. Explain how the addition of energy changes the movement of the molecules during each state of matter. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 3. In this lab, you saw how water changes state. Give 2 other examples of matter changing state. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ Conclusion The conclusion section needs to have five sentences: 1st sentence: Repeat the objective 2nd sentence: Describe what you did specifically in the lab to achieve the objective. 3rd sentence: State your hypothesis and use your data to explain if it correct or not and why. 4th sentence: Share what you learned. 5th sentence: This is a general summary of the lab. It ties into the first sentence of the purpose. _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________ _____________________________________________________________________________________________