preparation-of-esters1
advertisement

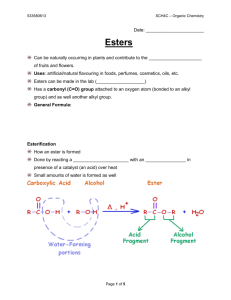
GRADE 12 PRESCRIBED EXPERIMENT 1: PREPARATION OF ESTERS NAME OF LEARNER : ……………………………………………………………. DATE : SCHOOL : ……………………………………………………………… TOTAL MARKS :50 1. Title Preparation and identification of Esters 2. Aim of the experiment * To synthesize/prepare esters, methylbutanoate and ethylbutanoate. * To write the chemical equations for the formation of methylbutanoate and ethylbutanoate using structural formulas. 3. Materials/Apparatus Equipment/ Glasswares 2 test tubes Water soluble marker Test tube rack Eye dropper 2 x 250 ml beakers Hot plate 10 ml graduated cylinder Thermometer Lab apron & Safety goggles Water bath Chemical / Reagents Methanol Ethanol Concentrated sulphuric acid Butanoic acid 4. Safety Precautions Concentrated sulphuric acid is a strong oxidizing agent and highly corrosive. It is dangerous and can burn skin, eyes, and clothing very badly. Avoid contact with skin or clothes. Wear gloves and proper eye protection when using this substance. Use only in a fume hood. Use exactly as directed. If it is spilled, wash immediately before the acid has a chance to cause a burn, and inform the instructor. Butanoic acid (all organic liquid acids) is toxic and corrosive to skin, eyes, and clothing. While working on this experiment, wear safety goggles, full face shield, gloves, and lab apron. Wash spills and splashes off your skin and clothing immediately, using plenty of water. All burners and other flames in the laboratory must be extinguished before you start this experiment. - Methanol is highly flammable. It is poisonous if swallowed. Its vapour is harmful to the eyes, lungs and skin and other organs. - Ethanol is flammable, poisonous and its toxic. Detect odours of these esters with caution because vapors produced may be harmful. Breathing the vapours of some of these esters can cause sore throat, dizziness, headache, and drowsiness. Hold the test tube 30 cm away and 15 cm below your nose. Waft a small amount of vapor or odour from the ester toward your nose, sniffing cautiously, once or twice. Do not breathe deeply while sniffing. Before leaving the laboratory, wash your hands thoroughly with soap and water. 1 5. Safety audit on: Ethanol Methanol Butanoic acid Sulphuric acid 6. Procedure/Method 1. Put on your lab apron and safety goggles. 2. Label the two test tubes 1 and 2 with your water soluble marker and place them in the test tube rack. 3. Into test tube 1, pour 5cm3 of methanol and in test tube 2, 5cm3 of ethanol and then add 6cm3 of butanoic acid as indicated in Table 1 below. Smell the mixture in each test tube as per safety precaution, above (waft!). Table 1 –Reagents for preparation of esters Test tube Constituents 5 cm3 methanol + 6 cm3 butanoic acid 1 2 5 cm3 ethanol + 6 cm3 butanoic acid 4. Add 4 drops of concentrated sulphuric acid to each test tube. 5. Pour about 150 ml of tap water in a 250 ml beaker. Place the test tubes in the water and heat the water on a hot plate to a temperature of about 60˚C – 75˚C. Leave the test tubes in the hot water bath for 15 minutes. 6. Cool the test tubes by immersing them in cold water in another beaker. 7. Add 5 ml of distilled water to each of the test tubes. Carefully note the odour of the contents of each of the test tubes in your copy of Table 2 in your notebook. Hold the test tube about 30 cm away from your nose and gently waft the vapour towards your nose without inhaling deeply. Each of the odours should be somewhat familiar to you. Alternatively, the contents of the test tube may be poured into a beaker half full of water and the odour above it detected carefully. 8. Dispose of all materials following the reagent disposal instructions. 9. Before leaving the laboratory, wash your hands thoroughly with soap and water. 7. Observations and recording Table 2 – Odours of Esters Test tube Aroma(smell) 1 2 Constituents Methanol + butanoic acid Ethanol + butanoic acid 8. Analysis and interpretation Identify the esters formed in relation to the odours smelt. Draw the structural formulae of the formation of the two esters. 9. Conclusion Make a list of the odours you were able to detect and the ester responsible for that odour. 2 10. Factors influencing results Mention all limitations and challenges in this experiment. 11. Conceptual questions (Questions related to the experiment) Questions 1. Write down the general equation for the reaction of an alcohol and a carboxylic acid (3) 2. Name the ester formed in each of the test tubes (Table 2, above). (4) 3. What is the function of sulphuric acid in these reactions? (2) 4. How would you dilute a concentrated sulphuric acid solution? (2) 5. Write 5 safety precautions that you took during this experiment (5) 6. Give four (4) uses of esters (4) 3 MARKING GUIDELINES RESULTS Test tube 1 2 Aroma Pineapple Apple Constituents Methanol + butanoic acid Ethanol + butanoic acid 1. Write down the general equation for the reaction of an alcohol and a carboxylic acid. R OH + HO O C O R R O C R + O H H (3) 2. 3. It acts as a catalyst. (2) 4. 5. Write 5 safety precautions that you took during this experiment Safety Precautions 6. (6) a. Butanoic acid is flammable, caustic and can cause burns. Do not inhale it. b. Pentanol and ethanol are flammable. c. Methanol is highly flammable and toxic if it is inhaled or swallowed d. Do not get in contact with the sulphuric e. Carry out the experiment in a well-ventilated place or in a fume cupboard. Any four (4) a. Making perfumes b. Making air fresheners c. Making drinks d. Making baking/ food colorants e. Used by animals for hunting f. Used to identify partners for mating (any five) TOTAL MARK : 50 4