Rate Law Practice 43
advertisement
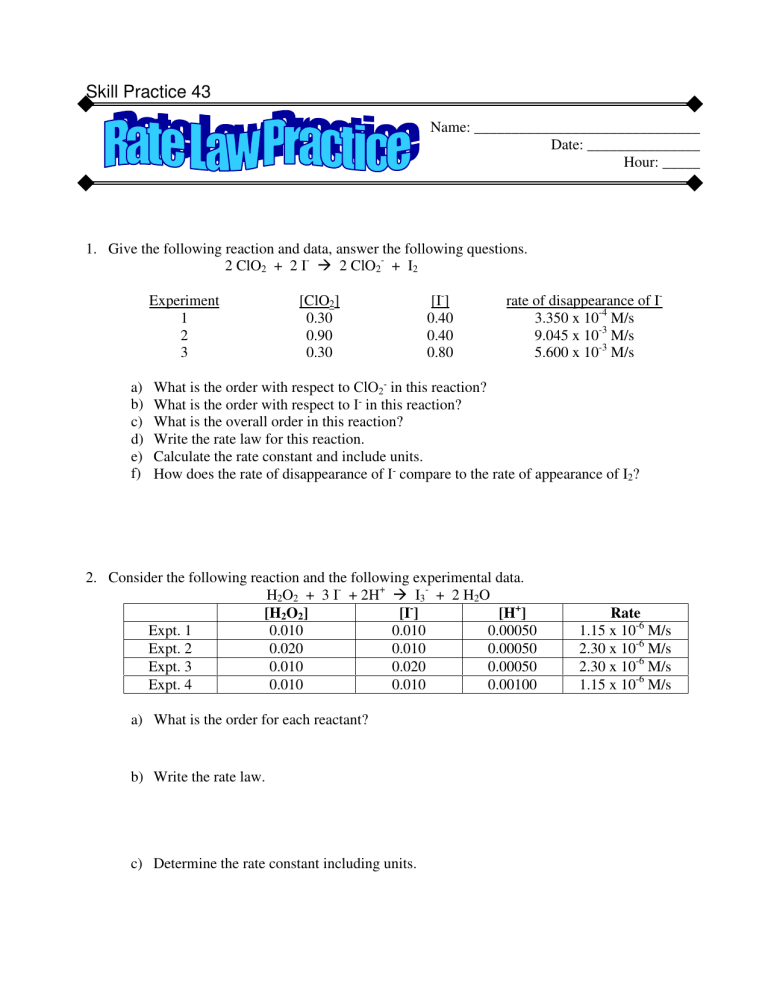
Skill Practice 43 Name: ______________________________ Date: _______________ Hour: _____ 1. Give the following reaction and data, answer the following questions. 2 ClO2 + 2 I2 ClO2- + I2 Experiment 1 2 3 a) b) c) d) e) f) [ClO2] 0.30 0.90 0.30 [I-] 0.40 0.40 0.80 rate of disappearance of I3.350 x 10-4 M/s 9.045 x 10-3 M/s 5.600 x 10-3 M/s What is the order with respect to ClO2- in this reaction? What is the order with respect to I- in this reaction? What is the overall order in this reaction? Write the rate law for this reaction. Calculate the rate constant and include units. How does the rate of disappearance of I- compare to the rate of appearance of I2? 2. Consider the following reaction and the following experimental data. H2O2 + 3 I- + 2H+ I3- + 2 H2O [H2O2] [I ] [H+] Expt. 1 0.010 0.010 0.00050 Expt. 2 0.020 0.010 0.00050 Expt. 3 0.010 0.020 0.00050 Expt. 4 0.010 0.010 0.00100 a) What is the order for each reactant? b) Write the rate law. c) Determine the rate constant including units. Rate 1.15 x 10-6 M/s 2.30 x 10-6 M/s 2.30 x 10-6 M/s 1.15 x 10-6 M/s




