Periodic Trends Worksheet
advertisement

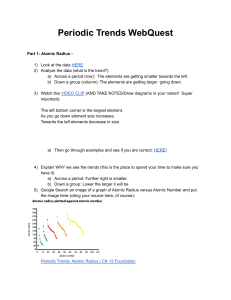
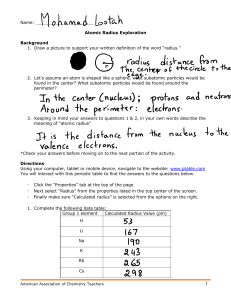
Name _________________________________________________________________________________ Date ________________ Periodic Trends Worksheet Directions: Use your notes to answer the following questions. Use COMPLETE SENTENCES for your answers. 1. Rank the following elements by increasing atomic radius: carbon, aluminum, oxygen, potassium. 2. Rank the following elements by increasing electronegativity: sulfur, oxygen, neon, aluminum. 3. Why do elements in the same family generally have similar properties? 4. table. a. b. c. Indicate whether the following properties increase or decrease from left to right across the periodic 5. What trend in atomic radius occurs down a group on the periodic table? What causes this trend? 6. What trend in ionization energy occurs across a period on the periodic table? What causes this trend? 7. a. b. c. d. e. f. Circle the atom in each pair that has the largest atomic radius. Al or B Na or Al S or O O or F Br or Cl Mg or Ca 8. a. b. c. d. e. Circle the atom in each pair that has the greater ionization energy. Li or Be Ca or Ba Na or K P or Ar Cl or Si 9. 10. a. b. c. d. e. f. atomic radius (excluding noble gases) first ionization energy electronegativity Define electronegativity. Circle the atom in each pair that has the greater electronegativity. Ca or Ga Br or As Li or O Ba or Sr Cl or S O or S