Burning Magnesium - An Exothermic Reaction
advertisement

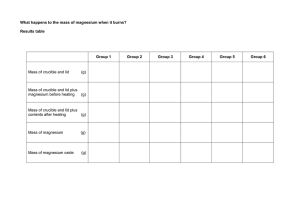
Burning Magnesium - An Exothermic Reaction When magnesium is in its metal form it will burn very easily in air. However, in order to start the reaction (the burning) the magnesium metal needs a source of energy. The flame provides a source of heat so that the magnesium metal atoms can overcome their activation energy. Activation energy is the minimum energy required in order for a chemical reaction to proceed. When the magnesium metal burns it reacts with oxygen found in the air to form Magnesium Oxide. A compound is a material in which atoms of different elements are bonded to one another. Oxygen and magnesium combine in a chemical reaction to form this compound. After it burns, it forms a white powder of the magnesium oxide. Materials 1. strip of magnesium metal ribbon - 2cm long 2. Lighter 3. Tripod 4. Gauze mat 5. Electronic scales 6. Crucible and lid 7. Bunsen burner Method 1. Weigh an empty crucible and lid. Record mass. 2. Place magnesium piece in crucible and replace lid. 3. Weigh the Magnesium + crucible using the scales and record mass. 4. Set up the apparatus as shown below. 5. Light the bunsen burner and place on heating flame. 6. Once the crucible is hot, gently lift the lid with the tongs a little to allow some oxygen to get in. You may see the magnesium begin to flare up. If the lid is off for too long then the magnesium oxide product will begin to escape. Don't let this happen. 7. Once the reaction has completed, allow the crucible to cool completely. 8. Re-weigh the crucible and record mass. Calculate the change in mass of magnesium 9. Compare and record results of other groups for reliability. Calculate the class average. CAUTION DO NOT LOOK DIRECTLY AT THE BRIGHT LIGHT. Briefly gaze at the light out of the corner of your eye. AIM: HYPOTHESIS: CALCULATIONS: (before burning) Mass of crucible and lid = Mass of crucible, lid and magnesium = Mass of magnesium = RESULTS: Mass of Mg before burning My group Group 1 Group 2 Group 3 Group 4 Group 5 Mass of Mg after burning Change in mass of Mg