10-Mole-Calculations-Multiple-Choice-Questions-and-Answers
advertisement

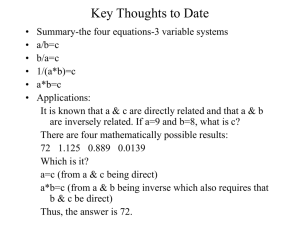
Mole Calculations Multiple Choice Questions 1 How many moles are there in 6 dm3 of oxygen at r.t.p.? A 0.25 moles ⃝ B 0.50 moles ⃝ C 0.60 moles ⃝ D 3.00 moles ⃝ 2 What is the total volume of gas remaining after 20 cm3 ethane are burned completely in 100 cm3 oxygen? All volumes are measured at the same pressure and the same temperature, which is above 100 °C. C2H6 + 3 ½ O2 2CO2 + 3H2O A 40 cm3 ⃝ B 100 cm3 ⃝ C 120 cm3 ⃝ D 130 cm3 ⃝ 3 What is the mass of 0.36 moles of ethanoic acid, CH3COOH? A 0.06g ⃝ B 21.60g ⃝ C 6.00g ⃝ D 36.00g ⃝ 4 What mass of H2SO4 is needed to produce 60cm3 of 0.25M solution? A 0.015g ⃝ B 6533g ⃝ C 0.0015g ⃝ D 1.47g ⃝ 5 What volume will be occupied by 88g of propane gas (C3H6) at r.t.p.? A 44 dm3 ⃝ B 24 dm3 ⃝ C 48 dm3 ⃝ D 12 dm3 ⃝ 6 What mass of sodium hydroxide needs to be dissolved to make 50cm3 of 2M solution? A 4g ⃝ B 0.1g ⃝ C 40g ⃝ D 25g ⃝ 7 What is the atom economy for the following reactions: C2H5OH + PCl5 C2H5Cl + POCl3 + HCl A 25.3% ⃝ B 64.5% ⃝ C 35.5% ⃝ D 29.0% ⃝ 8 What is the theoretical yield of PCl5 if 0.275g of PCl3 reacts with 0.142g of chlorine according to the equation? PCl3 + Cl2 PCl5 A 0.209g ⃝ B 0.142g ⃝ C 0.275g ⃝ D 0.417g ⃝ 9 What is the percentage atom economy for the production of methanol for the following reaction? CH3Br + NaOH CH3OH + NaBr A 135% ⃝ B 23.7% ⃝ C 32% ⃝ D 31.1% ⃝ 10 What is the theoretical yield if 9.2g of ethanol was reacted with an oxidising agent in excess and 2.1g of ethanol was produced according to the following equation? C2H5OH + [O] CH3CHO + H2O A 44g ⃝ B 0.2g ⃝ C 8.8g ⃝ D 24g ⃝ Mole Calculations Multiple Choice Questions ANSWERS: 1A 2D 3B 4D 5C 6A 7A 8D 9B 10 C