Biological-molecules
advertisement

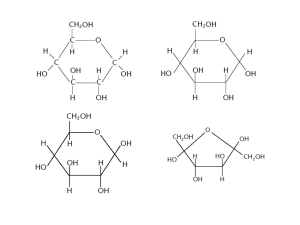
Carbohydrates: monosaccharides Learning objectives • • • Ref T&T p.8-9; BIR p.1-3 How are _____________ constructed? What is the structure of a _______________? How do you use ____________ reagent to test for reducing and ______________ sugars? Using a pencil and ruler, link the Latin prefix to the English translation. Tri Mono Poly Di One Two Three Many 1 Note the unusual feature of carbon atoms. 2 Explain why carbon-containing molecules are known as organic molecules. 3 Distinguish between a monomer and a polymer and include some examples. 4 Name the elements that make up most of the polymers in nature. 5 Name the three chemical elements contained in carbohydrates. 6 Describe the two main functions of carbohydrates in living organisms. 7 Arrange the different carbohydrates in size order, starting from the smallest on the left. CARBOHYDRATES 8 Glucose and fructose have the same chemical formula (_________). Explain how they can exist as two different monosaccharides. 9 Draw (and learn) the simplified version of - glucose. This is one of two molecules you need to be able to recall under exam conditions. Number the carbon atoms and state what makes it -glucose monosaccharide (and therefore not β-glucose monosaccharide). 1 Reducing sugars reduce Benedict’s reagent. All monosaccharides are reducing sugars and the disaccharides maltose and lactose are also reducing sugars - sucrose is not. 10 Explain why Benedict’s reagent turns red when heated with a reducing sugar. 11 Colour and label the diagram to show the results of the Benedict’s test. Using the instructions on p17 of this handout, complete the semi-quantitative Benedict’s test on the glucose solutions you have been given. Rank them in order from the weakest to the strongest. Record your results in a table below. 12 Answer the extension questions T&T, p9 1. (1 mark) 2. (2 marks) 3. (1 mark) 13 Glucose syrup is used in the production of many human foods. It is produced from starch in a series of enzyme-controlled reactions. One way of monitoring the progress of these reactions is to measure the amount of reducing sugar produced. (i) Describe a chemical test that would enable you to show that glucose syrup contained reducing sugar. ................................................................................................................................................... ................................................................................................................................................... .............................................................................................................................................. (2) (ii) Suggest how you could use this test to compare the concentration of reducing sugar in two solutions. ................................................................................................................................................... ................................................................................................................................................... .............................................................................................................................................. (2) 2 Carbohydrates: disaccharides and polysaccharides Learning objectives Ref T&T p.10-11; BIR p.3-4 How are ________________ linked together to form ______________? What is the test for _______________ sugars? What is the test for _________? How are ____________ _______________ linked together to form _________? 1. Group the carbohydrates into one of the three groups. Monosaccharide Disaccharide Polysaccharide Found in When ____ monosaccharides join forming a __________ bond, by a _____________ _________, two molecules are produced. A _____________________________________ 2. Identify the disaccharides that are formed when the following monosaccharides combine. • • • 3. -glucose + __________ __________. -glucose + __________ __________. -glucose + __________ __________. Complete Diagram 1 to show the formation of the disaccharide maltose from two -glucose monosaccharides. Label i) the names of the molecules, ii) the name of the reactions and ii) include the water molecule (use T&T p.10 if you need some help). Diagram 1: Formation of a glycosidic bond by a condensation reaction (removal of water) 4. Complete Diagram 2 to show the formation of two -glucose monosaccharides from the disaccharide maltose. Label i) the names of the molecules, ii) the name of the reactions and include the water molecule. Diagram 2: Breaking of glycosidic bond by a hydrolysis reaction (addition of water) 3 4 Maltose is formed by condensation of two -glucose molecules. Write the word and then the balanced chemical equation for the formation of maltose. 5 Carry out the test as instructed and note your results below (N.B. only complete the test for non-reducing sugars on those that did not give a positive test for reducing sugar). 6 Samples of six carbohydrate solutions of varying strength (%) were tested using Benedict’s reagent before boiling in acid. The degree of colour change was recorded as: 0 (no colour change) + (a little colour change) ++ (some colour change) +++ (maximum colour change) Some of the results are shown in the second column of Table 1. Sugar solution / % Result before boiling in acid Result after boiling in acid Glucose – 0.5 + + Glucose – 1.0 ++ ++ Glucose – 2.0 +++ +++ Fructose – 2.0 Maltose – 2.0 Sucrose – 2.0 Table 1: Results of testing six solutions with Benedict’s reagent before and after boiling in acid a) Using the same method of recording results, complete the ‘Result before boiling in acid’ column in Table 1 showing the expected results for fructose, maltose and sucrose. Another sample of each solution was boiled with dilute hydrochloric acid, neutralised using Sodium Hydrogen Carbonate powder and then tested with Benedict’s reagent. Some of these results are shown in the third column in Table 1. b) Using the same method of recording results, complete the ‘Result after boiling in acid’ column in Table 1 showing the expected results for fructose, maltose and sucrose. c) Explain your answers for maltose and sucrose. ................................................................................................................................................... ................................................................................................................................................... ................................................................................................................................................... ................................................................................................................................................... ................................................................................................................................................... ................................................................................................................................................... ................................................................................................................................................... 4 Polysaccharides Diagram 3 shows a polymer, made from the monomer _____-________, and is joined by __-__ __________ ______. These bonds are formed by _____________ reactions. This is a molecule of _______. It is large (200 – 100,000), tightly coiled and insoluble and is an ideal storage molecule. 1. Complete Diagram 3 to show the reactions that lead to the formation of this polysaccharide. Diagram 3: Formation of glycosidic bonds to produce the polysaccharide starch In this example, __ ______________ reactions have produced __ water molecules to produce the _______________ starch. A __________ reaction (addition of ________) reverses the reaction and splits the polysaccharide releasing __ _____________ molecules. 2. A polysaccharide containing 101 monosaccharides monomers contains ___ glycosidic bonds. 3. Lactose is a disaccharide sugar which can be broken down by the enzyme lactase into two monosaccharides, glucose and galactose. The reaction is shown below. lactose + water (a) lactase glucose + galactose The formula for galactose is C6H12O6. What is the formula for lactose? .......................... (1) (b) A solution containing the enzyme lactase was added to a lactose solution. Sample A was removed immediately. The remainder of the solution was incubated at 40 °C for one hour. Sample B was then removed after one hour. (i) Describe a chemical test you could carry out on sample A to show that lactose is a reducing sugar. ................................................................................................................................................................ ................................................................................................................................................................ .................................................................................................................................................................. (2) (ii) This chemical test was carried out on samples A and B. All experimental variables were the same in the testing of the two samples. Both tubes were left for ten minutes to allow the precipitate to settle. The diagram shows the result. Explain the results in the diagram ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... A B ............................................................................................................................... (3) (Total __/7) 5 Carbohydrates: starch, glycogen and cellulose Learning objectives Ref: T&T p.13-15; BIR p.4-6 How are _________ ________ arranged to form polymers of starch and _________? How are ____________ monomers arranged to form the polymer __________? How do the molecular structures of _______, _________ and ___________ relate to their function? An overview of starch, glycogen and cellulose Polysaccharide Monomer Glycosidic bond/s Structure Task 1: Starch Complete the diagram below showing the structure of a starch molecule. Complete and label the glycosidic bonds and number the carbon atoms that make up the glucose monomers. Use T&T, p.13 to draw a labelled diagram showing the helix structure of starch (amylose) Summarise, in no more than 30 words, the bullet points outline the reasons why starch it is so well suited as an energy storage molecule. 6 Task 2: Glycogen Only found in _________ and __________ cells (i.e. not _______ cells) and stored in small granules in _______ and liver. Glycogen is _________ in structure to________, but has shorter chains and is more highly____________. Because it is made from shorter chains of____________, with a 1-4 and _______________ bonds, it is more readily ____________ to α-glucose than starch, so provides an excellent source of ‘quick-release’ ________. Complete the diagram: i. numbering the carbon atoms and ii. completing and labelling the 1-4 and 1-6 glycosidic bonds. Task 3: Cellulose 1. Name the monomer that makes up cellulose. 2. Describe how the monomers in cellulose are arranged to produce a polymer that is good for support. Include: the importance of i) hydrogen bonds, ii) the positing of the hydroxyl (-OH) groups, iii) cross linkages and iv) a labelled diagram to support your explanation. 3. Make bullet point notes on the importance of cellulose in plant cell walls 4. Do microfibrils allow water to pass through them by osmosis? Explain the importance of this property. 7 Question time 1 Read the following passage and answer the questions that follow. Amylose is a straight chain molecule with 1–4 glycosidic bonds, whereas amylopectin is a branched molecule containing both 1–4 and 1–6 glycosidic bonds. Starch is made from a mixture of amylose and amylopectin. Like amylose, cellulose is a straight chain molecule with 1–4 glycosidic bonds but it consists of β- glucose molecules. Like amylopectin, glycogen is a branched molecule with 1–6 glycosidic bonds but it is more highly branched. a) What is a glycosidic bond and how is it formed? (2 marks) b) To what do the numbers 1 and 4 refer in a glycosidic bond? (1 mark) c) Explain how the arrangement of the monomers in cellulose are related to the function of plant cell walls. (3 marks) d) Draw a diagram to show the formation of the disaccharide cellobiose from two β-glucose monosaccharides. Label i) the molecules, ii) the name of the reaction and ii) include the water molecule. e) Explain how the presence of branching in amylopectin (a molecule similar to amylose but with a branched structure) and glycogen accounts for their role as ‘energy storage’ molecules. (2 marks) 2 Complete the table below by ticking the boxes to indicate which of the carbohydrate molecules contain α-glucose, β-glucose, 1–4 glycosidic bonds, 1–6 glycosidic bonds and which occur in plant cells. Amylose Amylopectin Glycogen Cellulose α - glucose β - glucose 1–4 glycosidic bonds 1–6 glycosidic bonds In plant cells 8 (5 marks) 3 The photomicrographs below show a leaf cell and a liver cell. a) Name the three carbohydrates A, B, and C on the diagrams above. (2 marks) b) Suggest three ways in which the structures of amylopectin and glycogen are similar. (2 marks) c) List three ways in which the structures of amylose and cellulose are different. (2 marks) d) State three properties of starch that make it suitable for its role as a storage molecule. (1 mark) e) State three properties of cellulose that make it suitable for its role in cell walls. 4 (1 mark) Like starch, which is digested to maltose and then glucose, cellulose is digested to cellobiose and then glucose. Because pandas are unable to digest cellulose it is just the cell contents of bamboo that supply them with their nutrients, so that pandas egest up to 80 per cent of what they consume. However, by harbouring bacteria that secrete cellulose-digesting enzymes, other herbivorous mammals lose far less of what they consume. a) What type of molecules are maltose and cellobiose? (1 mark) b) How do maltose and cellobiose differ? (1 mark) c) Name the chemical reaction involved in the digestion of starch and cellulose (1 mark) d) What allows herbivorous mammals to digest starch but not cellulose? (1 mark) 9 Lipids Learning objectives • • • • Ref: T&T p.16-17; BIR p.7-10 What are the _______ of __________? How does the ____________ of _____________ relate to their function? How does the structure of _____________ relate to their ____________? How is the ____________ of a lipid _____________? What is a lipid and what do they have in common? Lipids contain _________, _________ and ________. The proportion of ________ to carbon and hydrogen is smaller than in carbohydrates. They are made of a ___________ molecule and a few ______ ______. They are all insoluble in ______ and soluble in ______ (e.g. alcohol) The roles of lipids 1. Complete Table 1 detailing the roles of lipids (T&T, p.16): Energy source Provide _______ as many calories as the same mass of carbohydrate or ________ Waterproofing Plants and animals have a waxy cuticle. Mammals produce _______. Insulation Lipids are poor conductors, but good ________. Protection delicate organs like the _________ and heart have fat around them They form part of the _______ __________. The lipid here is a _____________. Table 1: The roles of lipids 2. Triglycerides are composed of one _________ molecule and ________________ that join via _ _____________ reactions. Label the molecule and complete the diagram to show the formation of the triglyceride from its constituents. 3. Diagrams 1 and 2 show two types of fatty acid that are commonly found in triglycerides. Use T&T, p.17 (and your observations) to label the diagrams and make brief notes detailing what makes these fatty acids different. Diagram 1:______________________ Diagram 2:______________________ 10 4. Draw a diagram of a phospholipid. Identify the polar (hydrophilic) head and non-polar (hydrophobic) tail. 5. The structure of a phospholipid molecule is different from that of a triglyceride. Describe how a phospholipid is different. ........................................................................................................................................... ........................................................................................................................................... ........................................................................................................................................... ............................................................................................................................. (2 marks) 6. Identify one consequence of a phospholipid having a polar head and a non-polar tail and explain where this consequence is put to good use. ........................................................................................................................................... ........................................................................................................................................... ........................................................................................................................................... ............................................................................................................................. (2 marks) 7. Fats and oils make up a group of lipids called _____________ which, when hydrolysed, form ___ ________ and ___ fatty acids. A fatty acid with more than one carbon-carbon double bond is called _______________. In a phospholipid the number of fatty acids is ___. Fatty acids are described as ____________ because they repel water. 8. Animals and plant seeds use lipids rather than carbohydrates as a long-term energy store. Suggest and explain one advantage of this. ........................................................................................................................................... ........................................................................................................................................... ........................................................................................................................................... ............................................................................................................................. (2 marks) 9. Dietary recommendations are that lipid intake should make up 30% of energy intake. The recommended energy intake for most men aged 19-49 is 10,500 kJ day–1. The energy content of lipid is 37.8 kJ g–1. Calculate the recommended lipid intake per day for these men. Show your working. Answer ................................................... g (1 mark) 11 Proteins Learning objectives • • • • Ref T&T, p.19-22; BIR p.10-13 How are amino acids linked to form __________ – the __________ structure of proteins? How are ___________ arranged to form the __________ structure and ___________ structure of a protein? How is the ____________ structure of a protein formed? How are proteins ____________? Proteins are three-dimensional ___________ whose monomers are the ___ different ______ _____ found in nature. Task 1: Draw (and learn) the general structure of an amino acid. This is another molecule you need to be able to recall under exam conditions. Annotate the molecule with the name and description of the groups that make it up. Task 2: Complete the diagrams below to show the formation of a dipeptide. In Diagram 1, circle the atoms that are removed and name the reaction. In Diagram 2, draw and label the bond that forms between the two amino acid monomers. Diagram 1 Diagram 2 Task 3: Draw and label a diagram below of a dipeptide and show how the peptide bond joining a dipeptide can be hydrolysed to form two amino acids. 12 Task 4: Label the diagram below using labels i-vii to show the four levels of organisation within a protein (use T&T, p.20 & 21 if you would like): i) Amino acids v) Quaternary structure ii) Primary structure vi) α-helix iii) Secondary structure vii) β-pleated sheet iv) Tertiary structure Task 5: i) Make notes on the importance of the primary sequence of a protein and explain how a change in one amino acid may lead to a change in the function of the protein. Include the following words in your answer: primary, secondary, alpha, beta, tertiary, hydrogen, ionic, disulphide, structure. ........................................................................................................................................................................ ........................................................................................................................................................................ ........................................................................................................................................................................ ........................................................................................................................................................................ ........................................................................................................................................................................ ........................................................................................................................................................................ ii) Use T&T, p.20 to draw the structure of the α-helix and label the bonds that hold it together. iii) The tertiary structure of a protein determines its function. List the features of the following bonds that maintain it: - Hydrogen bonds - Ionic bonds - Disulphide bridges iv) Note what is meant by the quaternary structure of a protein? ................................................................................................................................................................. ................................................................................................................................................................. 13 Task 6: Proteins perform many different roles in living organisms. Their roles depend on their molecular shape, which can be of two basic types. Make brief notes about: i) Fibrous proteins. ii) Globular proteins. Task 7: 1. Polypeptides can be made up from 20 different amino acids. A tripeptide is a polypeptide consisting of three amino acids. How many different tripeptides is it possible to make? 2. Give one way in which the formation of a peptide bond is similar to the formation of a glycosidic bond. Task 8: Read T&T, p.22 and then answer the green questions. 1. 2. 3. Practice exam questions 1. (a) __/14 marks Describe a biochemical test to find out if a substance contains a protein. ..................................................................................................................................... ..................................................................................................................................... ................................................................................................................................. (2) (b) The diagram shows the structural formulae of two amino acids. H2 N H O C C H OH H2 N H O C C OH CH 2 SH (i) Name one chemical element found in all amino acids, but not in monosaccharides. ....................................................................................................................... (1) 14 (ii) What type of chemical reaction occurs to form a dipeptide? ....................................................................................................................... (1) (iii) Draw the structural formula of the dipeptide formed when these two amino acids combine. (1) (Total __/5 marks) 2. (a) The diagram shows the molecular structure of a dipeptide which has been formed by joining two amino acids together. X H O C C CH 2 OH (i) H O N C C H CH 2 OH CONH 2 Give the formula of the chemical group present at position X on this molecule. .......................................................................................................................... (1) (ii) Draw a circle round the chemical bond which has been formed as the result of the condensation reaction between the two amino acids. (1) (iii) The table shows the chemical structure of the R–group in a number of different amino acids. Amino acid Alanine Asparagine Aspartic acid Glutamine Serine Structure of R-group CH3 CH2CONH2 CH2COOH (CH2)2CONH2 CH2OH Use the information in the table to name the two amino acids from which the dipeptide was formed. First amino acid ................................................................................................ Second amino acid ...................................................................................... (1) (b) The relative molecular mass (Mr) of a molecule is a measure of its size. (i) The mean Mr of an amino acid is 110. The Mr of a particular protein is 40 000. Calculate the number of amino acids that make up this protein. Show your working. Answer = .............................. (1) 15 (ii) Explain how it is possible for different proteins to have the same relative molecular mass. .......................................................................................................................... ....................................................................................................................... (1) (Total __/5 marks) 3. (a) The diagram shows the formula of a molecule of an organic compound. CH3 H2 N C COOH H (i) To which group of organic compounds does this molecule belong? .......................................................................................................................... (ii) Give one way in which this molecule differs from other compounds in the group. .......................................................................................................................... .......................................................................................................................... (2) (b) The table shows some of the organic compounds found in a bacterial cell. Compound Protein RNA DNA Lipid Glycogen Percentage of total dry mass Number of different types of molecule 55.0 20.5 3.1 9.1 2.5 1050 463 1 4 1 Glycogen and protein are both polymers. Explain why there can only be one type of glycogen molecule, but there can be many types of protein molecule. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................(2) (Total __/4 marks) 16 Testing for biological molecules Ref. BIR p.14-16 Test for starch (a polysaccharide) 1. Add 2 cm3 of the test solution to a test tube (or a few drops on a spotting tile). 2. Add several drops of dilute iodine in potassium iodide solution (Iodine solution) to the sample. 3. Note any colour change in a table of results. Positive result = blue-black colouration. For the following three tests, if the sample is not already in liquid form, grind/dissolve it in water. Benedict’s test - test for a reducing sugar, usually a monosaccharide. 1. Add 1cm3 of the test solution to a test tube. 2. Add 1cm3 of BENEDICT’S REAGENT (take care with Benedict’s reagent!). 3. Agitate the tube to mix the solutions. 4. Add heat by placing the test tube in a hot thermostatically controlled water bath for 5 min. 5. Note the colour of any precipitate formed in a table of results. Test for a non-reducing sugar - usually a disaccharide Do not do this test IF you obtained a positive result when testing for a reducing sugar. 1. Add 1cm3 of DILUTE HYDROCHLORIC ACID (care!) to 1cm3 of the test solution. 2. Place the test tube in a (gently boiling) thermostatically controlled water bath. Continue boiling for 5 minutes. (This hydrolyses the sugar into its constituent monosaccharides). 3. Neutralise the solution with a little SODIUM HYDROGEN CARBONATE powder - test with pH paper. 4. Add 1cm3 of BENEDICT’S REAGENT (take care with Benedict’s reagent!). 5. Add heat by placing the test tube in a hot thermostatically controlled water bath for 5 min. 6. Note the colour of any precipitate formed in a table of results. The Biuret test - test for a protein. 1. Add a little SODIUM HYDROXIDE (Caustic!) solution to a solution of the protein in a test tube. 2. Carefully add a few drops of Biuret (1% copper sulphate solution) down the side of the test tube. 3. Look for a blue ring at the surface of the solution. 4. Agitate the mixture gently 5. Note any colour change in a table of results. A positive result = violet / lilac / purple coloration (NOT blue!) The Emulsion test - test for a lipid Precaution – the tubes must be completely dry and free from grease 1. If the test substance is not in liquid form, crush/grind it with ethanol/alcohol. 2. Place 2 cm3 of the test solution into a test tube and add 5 cm 3 of ethanol (flammable - care!) 3. Place a rubber bung on the end of the tube and invert the tube several times so that the contents are thoroughly mixed. Then allow the mixture to settle. 4. Pour the upper layer into 5 cm3 distilled water in another test tube 5. Note any colour change in a table of results. A positive result = emulsion forms / cloudy-white Task: Complete the activities / questions on BIR, p.14-16. 17