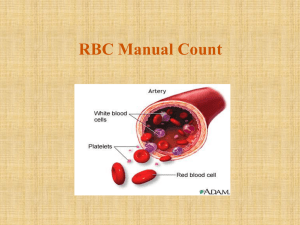
RBC Manual Count
advertisement

RBC Manual Count Prepared by Yasser El-dahdouh HEMOCYTOMETER Hemo: blood Cyto: cell Meter: measurement/counter Thus, it is an instrument used to count the blood cells. types of haemocyto metry Double Neubauer Ruled • Metallized Counting Chamber Petroff-Hausser Counting • Chamber types.. C-Chip Disposable • Hemacytometer Cellometer™ – The Disposable Cell Counting Chamber *** Hemacytometers a precision-made slide for performing manual cell counts with the aid of a microscope. *** Hemacytometers are used when…… 1- automated cell counters and hematology analyzers are unavailable 2- blood cell counts are extremely low 3- to get a cell count for body fluids (spinal fluid, joint fluid, semen counts, and other bodily fluids) The most commonly used hemacytometer is the Neubauer chamber. It includes: Neubauer’s slide (a Cover slip (b RBC pipette (c WBC pipette (d NEUBAUER’S SLIDE ** It is the name given to a thick glass slide. In the centre of the slide, there is an H- shaped groove. On the two sides of the central horizontal bar, there are scales for counting the blood cells. ** The depth of the scales is 1/10mm or 0.1mm. ** Each scale is 3mm wide and 3mm long. ** The whole scale is divided into 9 big squares. ** Each square is 1mm long and 1mm wide. Differences Between RBC AND WBC Pipette. RBC pipette WBC pipette 1) It has a red bead It has a white bead 2) It has It has graduations upto graduations upto mark 101 mark 11 3) Size of bulb is larger Size of bulb is smaller RBC PIPETTE WBC PIPETTE DILUTION FACTORS For CELL counting Blood is filled till mark 0.5 and fluid is then filled till mark 101 or 11. Both are thoroughly mixed. The numbers written on the chamber mean that the space between the chamber and the cover slip is 0.100 mm and that the smallest square on the grid has an area of 0.0025 mm2. Clean **The numbers written on the chamber mean that the space between the chamber and the cover slip is 0.100 mm and that the smallest square on the grid has an area of 0.0025 mm2. ** Clean the Neubauer chamber and the cover slip with 70% ethanol. With the microscope, using a 4x objective, identify the nine main squares of the counting chamber delimited by three lines each as shown in the following image: ** Now change to 10x objective and focus one of the 9 main squares. **The counting is performed in the area delimited by three lines. ** Cells that touch the upper and left border are counted (black color) whilst cells that touch the right and lower border are not counted (red color). The smallest square has an area of 0.0025 mm2 therefore each main square has an area of 0.04 mm2 (0.0025 x 16 = 0.04). Erythrocyte Count • Is the number of erythrocytes per micro litter of blood. • Normal ranges: – Male – Female – New born 4.2 – 5.4 3.6 – 5.0 5.5-6.5 10^6/µL 10^6/µL 10^6/µL • Erythrocyte count increased in case of polycythemia and decreased in anemia. Erythrocyte Count • Principle: – In order to facilitate RBCs count a specified volume of blood is diluted with a specified volume of isotonic fluid. – Red cell diluting fluid must be: • • • • anti-coagulant anti-hemolysis. anti-aggregation. anti-Rouleaux. preserve RBC shape. Erythrocyte Count Diluting fluid: • One of the following solutions may be used: 1. Isotonic saline: – 0.85% sodium chloride (NaCl) in distilled water. ** Dilution with normal Saline: Maintain the normal disk shape of the RBC Prevents autoagglutination 2- Hayam’s solution: – Sodium Sulphate – Sodium Chloride – Mercuric Chloride – Distilled Water 10 g. 2g. 0.25 g. 100ml 3- Gower’s solution: Sodium Sulphate Glacial acetic acid Distilled water 4- Citrate-formalin solution: Tri-sodium Citrate Formalin. 12.5 g. 33.3 ml 100 ml. • Note: – In certain conditions, Hayam’s solution may cause clumping of RBCs and Rouleaux formations, while Gower's solution prevents these problems • Sample: – Whole blood using EDTA or heparin as anticoagulant. Capillary blood may also be used. • Equipments: 1. (Pipettes) used one of the following: • • Thoma pipette (RBCs) Micropipette –20l is the desired volume. 2. Improved Neubauer chamber with the cover slips. 3. Conventional light microscope. 4. Clean gauze. Procedure: 1. Dilution of the blood: – Micropipette (20) 1:201 dilution. • Pipette 4.0ml of diluting fluid into a tube • Pipette 20l of will mixed anticoagulated whole blood to the tube. – Thoma red count pipette. • Draw the blood up to exactly the 0.5 mark and dilute to the 101 mark. • Mix continuously for 2-3 minutes. 2. Load the cleaned hematocytometer. 3. Place the hematocytometer on the microscope stage, lower the condenser. Procedure: 4. Focus with x10 objective lens on the large central square. This square is ruled into 25 small squares, each of which is further divided into 16 smaller squares, of the 25 squares, only the four corner squares, and one middle square are used to count RBCs. 5. Switch to 40 objective lens, and start counting in the five designated squares. • The large center square is used for RBC and PLT counts. • The center square is divided into 25 smaller squares, which are each subdivided into 16 squares. • Only 5 of the 25 squares are used to count red blood cells. • These 5 are usually the 4 outer squares and the inner most center square. • The entire large center square is used to count platelets. Preparation of the slide Step 1 Place the cover slip Step 3 Load Step Step 2 Dilute the sample 1:200 Step 4 Count in the small 5 center squares for RBC Calculations: • Total RBC Count = – • N x Dilution Factor x Volume Correction Factor Where: – – • N = the total number of red cells counted in the counting chamber. Dilution 1: 200 • Dilution Factor = 200 Counted Volume: – – – • • Each counted square has a volume of 0.2 X 0.2 X 0.1 = 0.004 5 squares volume = 5 X 0.004 = 0.02 cumm Volume correction factor = 1/0.02 = 50 So, Total RBC count = – N X 200 X 50 = N X 10.000 Sources of errors Falsely high counts:* collection of blood from the area where there is hemoconcentration. * improper pipetting of blood as well as the fluid. * improper mixing. *Errors in calculation. Falsely low counts:* Blood dilution with tissue fluid due to edem. * improper pipetting and dilution. * errors in calculation. Discussion 1. In certain conditions, such as polycythemia, the red blood cell count may be extremely high, which makes it difficult to obtain an accurate count. In this instance, make a larger dilution of blood. For a 1:301 dilution, add 20L of whole blood to 6.0mL of diluting fluid. 2. For a patient who has severe anemia and in whom the RBC is low, make a 1:101 dilution by adding 20L of whole blood to 2.0mL of diluting fluid. Discussion 3. Make certain the pipettes, hemacytometer, and cover glass are free from dirt, lint, and dried blood. Ensure that the diluting fluid is free from blood and other contamination. 4. RBC takes longer to perform than a WBC because of the larger number of cells. Therefore, proceed as quickly as possible once the cells have settled. Drying of the dilution in the counting chamber causes inaccuracies in the final cell count. 5. The range of error for a manual RBC is generally about 10 to 20%