Analysis of Impurities of Ru Dye (N719) for Dye
advertisement

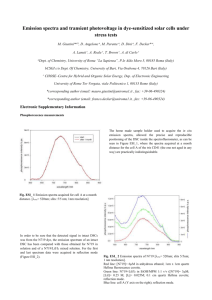
LAAN-A-LM-E046 Liquid Chromatography Mass Spectrometry No.C75 Analysis of Impurities of Ru Dye (N719) for Dye-Sensitized Solar Cells Solar cells are classified into several types, including a crystalline silicon type, a thin-film silicon type, a compound system type (CIGS, etc.), and the organic system type (organic thin-film type and dye-sensitized type), etc. Of these, about 90 percent of the solar cells being manufactured now are crystalline silicon solar cells. However, due to their manufacturing cost and the instability of a high-purity silicon supply, research and development of the next generation of dyesensitized solar cell is being promoted. The dye-sensitized solar cell is based on a system that generates electricity using dyes that are excited by light. This design has the advantages of high flexibility in determining color and shape, as well as low manufacturing cost. However, a variety of problems with this approach must first be addressed, including a solar conversion efficiency that is only about 1/3 that of the crystalline silicon type, and reliability (endurance), etc. In particular, even a minute amount of impurity in the dye will have a very adverse affect on the solar conversion efficiency. Here we introduce an example of the separation and qualitative analysis of impurities in the widely used dye, Ru N719, using the LCMS-2020. *The Ru N719 dye was kindly provided by Dr. Liyuan Han of the NIMSAdvanced Photovoltaics Center in Ibaraki, Japan. n Flow Injection Analysis of N719 Using LCMS-2020 N719 is a dye with improved solar conversion efficiency which is derived from N3 by the bonding of tetrabutylammonium (TBA) at 2 of the carboxyl sites of the N3 dye. Fig. 1 shows the structure of N719. After dissolving the N719 sample in ethanol, ESI measurement was conducted. Fig. 2 shows the positive and negative mass spectra obtained. In the ESI positive mode, the tetrabutylammonium molecular ion was detected, while deprotonated molecules of compounds bonded with 0 to 3 tetrabutylammonium groups, as well as doubly-charged ions, etc. were detected using the ESI negative mode. ○0 tetrabutylammonium detected N+ COOH N719 COOH HOOC OOC COOH N N SCN Ru SCN N Ru N NCS COO- C58H86N8O8RuS2 Exact Mass : 1188.51 Mol. Wt. : 1188.55 N+ COOH N N - NCS N3 N N C26H16N6O8RuS2 Exact Mass : 705.95 Mol. Wt. : 705.64 COOH ○1 tetrabutylammonium detected C42H51N7O8RuS2 Exact Mass : 947.23 Mol. Wt. : 947.10 N3 [TBA] ○3 tetrabutylammonium detected C74H121N9O8RuS2 Exact Mass : 1429.78 Mol. Wt. : 1430.01 N3 [TBA]3 Fig. 1 Structure of N719 5.0 Inten. (× 1,000,000) 242.3 ESI (+) Tetrabutylammonium (TBA) 4.0 N+ 3.0 2.0 C16H36N+ Exact Mass : 242.28 Mol. Wt. : 242.46 1.0 0.0 1.5 1.0 0.5 0.0 250 Inten. (× 1,000,000) 500 750 364.3 Doubly-charged 351.8 ion of N3 Doubly-charged ion of N3 [TBA] 263.0 Doubly-charged 472.4 ion of N719 N3 593.6 705.0 635.5 197.0 250 500 750 1000 1250 1500 m/z ESI (--) 824.7 N3 [TBA] 946.4 1000 Fig. 2 FIA Mass Spectra of N719 1067.5 N719 1187.7 1250 N3 [TBA]3 1428.9 1500 m/z No.C75 n Analysis of Impurities of N719 Using LCMS-2020 By conducting the analysis under acidic conditions, separation of N3 dye impurities having different structures at the X and Y sites was achieved. Fig. 3 shows the LC and MS chromatograms, and Fig. 4 shows the mass spectra at peaks A, B, F, and N3. 6.0 (× 10) N3 COOH 5.0 HOOC 4.0 3.0 COOH N Ru X 1.0 0.0 0.0 (× 10,000,000) 2.5 F N N 2.0 2.5 Separation of N3 and compound F, which is difficult using the typical ODS column, was easily achieved using these conditions, demonstrating the ease with which quality control can be conducted. N Y COOH A 5.0 B 7.5 C D E 10.0 12.5 2.0 15.0 min 1: TIC F 1.5 A 1.0 B Tetrabutylammonium C G 0.5 0.0 0.0 2.5 5.0 7.5 10.0 PDA Ch1 (532 nm) 12.5 15.0 min 1: 689.00 (28.85) 1: 674.95 (100.00) 1: 706.95 (24.20) 1: 242.10 (1.11) 1: 219.00 (5.81) Fig. 3 Chromatograms of N719 in Ethanol Solution A Inten. (× 10,000) 3.0 186.1 2.0 60.0 1.0 131.1 250 B Inten. (× 100,000) 112.3 0.5 83.0 0.0 689.0 237.2 328.7 0.0 1.0 [M+H]+ 531.5 500 750 m/z X: -NCS Y: -CN 648.0 [M+H]+ 707.0 5.0 750 0.0 m/z X: -SCN Y: -NCS 421.6 250 N3 Inten. (× 100,000) 500 5.0 2.5 [M+H]+ 675.0 500 7.5 2.5 648.0 186.0 250 X: -NCS Y: -CH3CN F Inten. (× 10,000) 0.0 750 648.0 [M+H]+ 707.0 m/z X: -NCS Y: -NCS 186.4 250 500 750 m/z Fig. 4 ESI(+) Mass Spectra of Impurities A, B, F and N3 Table 1 Analytical Conditions for LC/MS Column Mobile Phase A Mobile Phase B Gradient Program Flow Rate Injection Volume Column Temperature : Phenomenex Fusion RP (150 mmL. × 2.0 mm I.D., 4 μm) : 1 % formic acid - water : acetonitrile : 5 %B (0 min) - 75 %B (15-20 min)- 5 %B (20.01 - 30 min) : 0.2 mL/min : 2 μL : 25 °C SHIMADZU CORPORATION. International Marketing Division 3. Kanda-Nishikicho 1-chome, Chiyoda-ku, Tokyo 101-8448, Japan Phone: 81(3)3219-5641 Fax. 81(3)3219-5710 Cable Add.:SHIMADZU TOKYO Probe Voltage : +4.5 kV (ESI-Positive mode), -3.5 kV (ESI-Negative mode) : 1.5 L/min Nebulizing Gas Flow : 10 L/min Drying Gas Flow : 250 °C DL Temperature Block Heater Temperature : 450 °C : default values DL, Q-Array Voltages