Quantum Physics Slides - Little Shop of Physics
advertisement
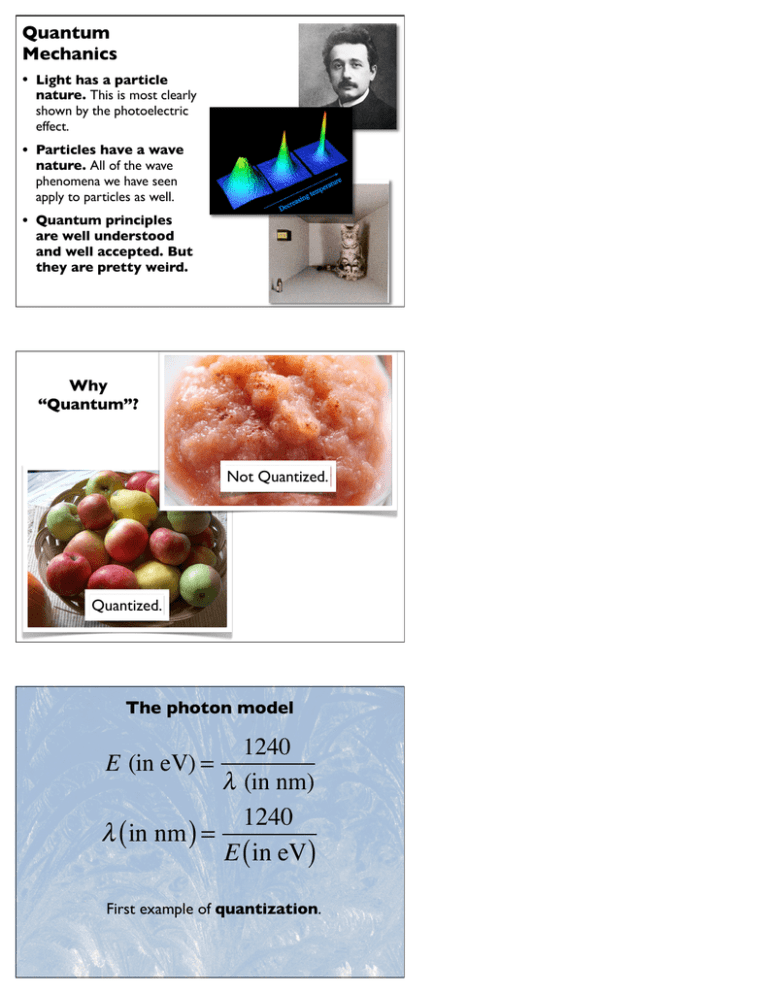
Quantum Mechanics • Light has a particle nature. This is most clearly shown by the photoelectric effect. • Particles have a wave nature. All of the wave phenomena we have seen apply to particles as well. • Quantum principles are well understood and well accepted. But they are pretty weird. Why “Quantum”? Not Quantized. Quantized. The photon model 1240 λ (in nm) 1240 λ ( in nm ) = E ( in eV) E (in eV) = First example of quantization. Creating X rays If an electron is accelerated through a 5.0 kV potential difference, what is the maximum photon energy of the resulting x ray? What is the wavelength? One electron. One photon. Photon Production A particular species of bioluminescent copepod (a small marine crustacean, typically a few mm in length) emits blue light at a peak wavelength of 490 nm. In a typical flash lasting 2.4 s, the copepod emits 1.4 x 1010 photons. • What power does this correspond to? • What is the intensity at a distance of 10 m? hc λ h = 6.62 × 10 −34 J ⋅s Ephoton = P = ΔE Δt I = Psource 4π r 2 Can You See a Single Photon? At the wavelength corresponding to the maximum sensitivity of the human eye, 510 nm, the limit of sensitivity of the darkadapted eye has been shown to be correspond to a 100 ms flash of light of total energy 240 eV. (Weaker flashes of light may be detected, but not reliably.) a) What is the energy of a single photon at this wavelength? b) How many photons does the flash contain? c) If 60% of the incident light is lost to reflection and absorption by tissues of the eye, how many photons reach the retina? The light from the flash covers well over 500 rod cells. d) So, can you see a single photon? Quantum Concept #1: EM Waves have a particle nature The Photoelectric Effect Light Window I Ammeter Cathode Anode A f DV I 0 f0 There is a threshold frequency. Above it, electrons are emitted. Below it, not so much. Just Checking In the photoelectric effect experiment, why does red light not cause the emission of an electron though blue light can? The photons of red light don’t have sufficient energy to eject an electron. Red light contains fewer photons than blue, not enough to eject electrons. The electric field of the red light oscillates too slowly to eject an electron. The red light doesn’t penetrate far enough into the metal electrode. The Photoelectric Effect Light Window I Intense light Weak light Ammeter Cathode Anode A DV 2Vstop 0 I DV Changing the accelerating voltage changes the current. But only within certain limits. Just Checking In the photoelectric effect experiment, increasing the accelerating voltage from 3.0 V to 5.0 V does not increase the current. How can we explain this result? The resistance of the tube changes as well. 28.2 The electrons are already at their maximum speed. The Photoelectric Effect 913 makes28.8 all the FIGURE A swimming pool analogy g pool. Water3.0 V Increasing the electrons reach the of electrons in a metal. m. To remove voltage doesn’t anode, so increasing change the electron orce of gravity, The minimum energy to remove a voltage drop causes of water no from the pool is mgh. kinetic energy. er molecule—change. e. Removing a h . Water Removing this drop etal. To extract takes more than the ugh to escape. minimum energy. k function of The Work Function e more energy work functions How much it “costs” to release an electron. 60 * 10-19 J.) This varies with the electrode. e 28.6. When TABLE 28.1 The work functions tic energy. An for some metals , so it emerges Element E0 (eV) n energy E0 is Potassium 2.30 ossible kinetic ns. FIGURE 28.9 and the anode = 0, there will e anode, creat- Sodium 2.75 Aluminum 4.28 Tungsten 4.55 Copper 4.65 Iron 4.70 Gold 5.10 Think About It. Light Window Ammeter Cathode Anode DV A I 5.0 eV photons strike an electrode with work function 3.0 eV. a. What is the kinetic energy of emitted electrons? b. What potential is needed to reduce the current to zero? Just Checking. Monochromatic light shines on the cathode in a photoelectric effect experiment, causing the emission of electrons. If the frequency of the light stays the same but the intensity of the light is increased, the emitted electrons will be moving at a higher speed. there will be more electrons emitted. both A and B are true. neither A nor B are true. Just Checking. Monochromatic light shines on the cathode in a photoelectric effect experiment, causing the emission of electrons. If the intensity of the light stays the same but the frequency of the light is increased, the emitted electrons will be moving at a higher speed. there will be more electrons emitted. both A and B are true. neither A nor B are true. The Details. Light of wavelength 400 nm illuminates a potassium electrode (work function 2.3 eV). a. What is the photon energy? b. What is the energy of the emitted electron? c. What is the stopping potential? Light Window Ammeter Cathode DV Anode A I 7. Metal surfaces on spacecraft in bright sunlight develop a net electric charge. Do they develop a negative or a positive charge? Explain. 8. Metal 1 has a larger work function than metal 2. Both are What’s The Fizics? Quantum Concept #2: Particles have a wave nature. Diffraction and Interference Diffraction Diffraction and Interference Double Slit Interference Pattern Viewing screen Incident laser beam Longer wavelength means bigger spacing. Grating Interference Pattern Screen y y2 m52 y1 m51 0 m50 2y1 m51 2y2 m52 Grating u2 u1 Dr between these paths is exactly 2l (m 5 2). Appearance of screen L Particles have a Wave Nature λ= h h = p mv De Broglie wavelength for a moving particle Particle or Wave? m λ Localized. Wavelength of a squirrel running at 3 m/s: 1x10-33 m Smeared out. Particle or Wave? In a television set, an electron is accelerated by a voltage of 150 V. a. What is the kinetic energy of the electron? b. What is the speed of the electron? c. What is the De Broglie wavelength? Does this matter? Size of a hydrogen atom Orbitals 0.1 nm Looking Deeper Electron microscope view of pigment molecule. Quantum Concept #3: The wave nature of particles leads to quantization. Particles have a wave nature. So... Particle: L m v Wave: L ...the possible states are quantized. The Crux of the Quantum Biscuit Photons have a particle nature. Their energy is quantized. It comes in chunks of a particular size. Particles have a wave nature. Confining them restricts them to certain energy states. The energy of a confined particle is quantized. It is restricted to certain values. The wave nature of particles leads to quantized energy levels for electrons in atoms. Only certain transitions are possible. Energy Energy 160 eV n54 90 eV n53 n54 160 eV 90 eV 40 eV n52 40 eV 10 eV 0 n51 10 eV 0 DEsystem 5 |E3 2 E1| 5 80 eV DEsystem 5 |E1 2 E2| 5 30 eV n53 n52 n51 Ground state Energy levels for a particle in a 0.10-nm-long box Possible transitions for a system with these energy levels 2 En = 1 ⎡ hn ⎤ h2 2 = n 8mL2 2m ⎢⎣ 2L ⎥⎦ n = 1, 2, 3, 4... What is the maximum photon energy that could be emitted by the quantum system with the energy level diagram shown below? The minimum? The Details. Light of wavelength 400 nm illuminates a potassium electrode (work function 2.3 eV). a. What is the photon energy? b. What is the energy of the emitted electron? c. What is the stopping potential? Light Window Ammeter Cathode DV Anode A I Ocean water is most transparent at wavelengths of 470 nm, so bioluminescent creatures emit light at approximately this wavelength. Firefly squid use ATP to provide the energy for this reaction. Metabolizing one molecule of ATP releases 0.32 eV. How many molecules of ATP must be metabolized to produce one photon of blue light at 470 nm? In a photoelectric effect experiment, light of wavelength 620 nm shines on a cathode with a work function of 1.8 eV. • What is the speed of the emitted electron? • What anode voltage will stop current in the tube? Particles have a Wave Nature λ= h h = p mv De Broglie wavelength for a moving particle . Electrons are accelerated from rest through an 8000 V potential difference. By what factor would their de Broglie wavelength increase if they were instead accelerated through a 2000 V potential? . Can an electron with a de Broglie wavelength of m pass Electron moving more slowly: Wavelength is longer. Ratio reasoning. K = ΔU e K = 12 mv 2 λ= h h = p mv The wave nature of particles leads to quantization. L m v L 2 1 ⎡ hn ⎤ h2 2 En = = n 8mL2 2m ⎢⎣ 2L ⎥⎦ n = 1, 2, 3, 4... Allowed energies for particle in a box The wave nature of particles leads to quantized energy levels for electrons in atoms. Only certain transitions are possible. Energy Energy 160 eV n54 90 eV n53 n54 160 eV 90 eV 40 eV n52 40 eV 10 eV 0 n51 10 eV 0 DEsystem 5 |E3 2 E1| 5 80 eV DEsystem 5 |E1 2 E2| 5 30 eV n53 n52 n51 Ground state Energy levels for a particle in a 0.19-nm-long box Possible transitions for a system with these energy levels What energy photons could be emitted by the quantum system sketched below? Electrons of the bonds along the chain of carbon atoms in this dye molecule are shared among the atoms in the chain, but are repelled by the nitrogen-containing rings at the end of the chain. What is the longest wavelength of visible light this molecule will absorb? 0.85 nm If the length of the chain is increased, how will this affect the wavelength of the light absorbed by the dye? Ratio reasoning. 2 1 ⎡ hn ⎤ h2 2 En = = n 8mL2 2m ⎢⎣ 2L ⎥⎦ n = 1, 2, 3, 4... Changing Scale The diameter of a typical atomic nucleus is about 10 fm. (1 fm is 1x10-15 m.) What is the kinetic energy, in MeV, of a proton with a de Broglie wavelength of 10 fm? Heisenberg uncertainty principle ∆ x ∆px Ú h 4p Δx Electrons & Atoms An electron is associated with a particular atom. This limits it to an uncertainty in position of about 1 nm—it’s somewhere within this range. What uncertainty in speed does this imply? One Photon, One Electron 66. ||| A silicon solar cell behaves like a battery with a 0.50 V terminal voltage. Suppose that 1.0 W of light of wavelength 600 nm falls on a solar cell and that 50% of the photons give their energy to charge carriers, creating a current. What is the solar cell’s efficiency—that is, what percentage of the energy incident on the cell is converted to electric energy? 67. |||| What is the kinetic energy in eV of an electron whose de Bro- r they provide us with a basis for understanding these elusive but most fundaconstituents of nature. This two-sided point of view is called wave–particle A spherical virus has a diameter of 50 nm. It is contained over two hundred years, scientists and nonscientists alike felt the clockwaves obey the principle of superposition and 0.0001 exhibit interference. This inside a long, narrow cell of length m.that niverse of Newtonian physics was a fundamental description of reality. But particle dichotomy seemed obvious until physicists encountered irrefutable particle duality, along with Einstein’s relativity, undermines the basic assumpWhat uncertainty does this imply for the velocity of the ce that light sometimes acts like a particle and, even stranger, that matter f theacts Newtonian worldview. Theofcertainty andAssume predictability of classical virus the length the cell? the virus has a mes likealong a wave. have given way to a new understanding of the universe in which chance and might at first think that light and matter are both a wave and a particle, but densityroles—the equal touniverse that ofof water. inty play key quantumofphysics. a doesn’t quite work. The basic definitions particleness and waviness are ly exclusive. Two sound waves can pass through each other and can overlap ucenature a larger-amplitude wave; two baseballs It isfrequent more profitable ual of a buckyballsound Treating atomic-level structurescan’t. involves lude that light arecarbon neither a wave northe a particle. ween particle and and wavematter views. 60 atoms can create molecule At the micromed left, knownand as C buckminsterfullerene. The scanning electron scaleatof atoms their physical scale not directly accessible 60, orconstituents—a ope of a C60 molecule on theof right is a particle-like viewturn of the iveimage senses—the classicalshown concepts particles and waves out to be sime with individual carbon atoms clearly visible. The C60 molecule, though we limited to explain the subtleties of nature. e a picture of it—showing the atoms that make it up—also has a wave nature. A ough matter and light have both wave-like aspects and particle-like aspects, C 60 sent through a grating will produce a diffraction pattern! ow us only one face at a time. If we arrange an experiment to measure a ke property, such as interference, we find photons and electrons acting like not particles. An experiment to look for particles will find photons and eleccting like particles, not waves. These two aspects of light and matter are nance imaging mentary to each other, like a two-piece jigsaw puzzle. Neither the wave nor manent mag- where m = 1.41 * 10-26 J/T is the known value of the proton’s ticle model alone provides an adequate picture of light or matter, but taken t of electrons magnetic moment. FIGURE 28.25 shows the two possible energy r they provide us with a basis for understanding these elusive but most fundaons also have states. The magnetic moment, like a compass needle, “wants” to constituents for magnetic of nature. This two-sided point of view is called wave–particle align with the field, so that is the lower-energy state. But a quantum compass is different. FIGURE 28.25 Energy levels for a proton in a magnetic field. over years, scientists and nonscientists alike felt that the clockaligntwo withhundred a . . . which correspond of to reality. But niverse NewtonianQuantum physics was a fundamental description on. This of isn’t mechanics limits two possible orientations, particle duality, along with Einstein’s relativity, undermines the basic assumpntum physics the proton to two aligned with or opposite There only f theare Newtonian worldview. The certainty and predictability of classical possible energies ... the magnetic field. orientations— have given way to a new understanding of the universe in which chance and Energy r inty play key roles—the universe of quantum physics. B E2 5 1mB field 0 eual thenature field of a buckyball Treating atomic-level structures involves frequent ween particle and wave views. 60 carbon atoms can create the molecule E 5 2mB med at left, known as C60,1or buckminsterfullerene. The scanning electron ope image of a C60 molecule shown on the right is a particle-like view of the −26 µproton carbon = 1.41× 10clearly J/T e with individual atoms visible. The C60 molecule, though we e a picture of it—showing the atoms that make it up—also has a wave nature. A C60 sent through a grating will produce a diffraction pattern! • What is the photon energy corresponding to a spin flip for a proton in a 1.0 T magnetic field? • What frequency does this correspond to? What type of EM wave is this? nance• imaging manent magt of electrons ons also have for magnetic where m = 1.41 * 10-26 J/T is the known value of the proton’s magnetic moment. FIGURE 28.25 shows the two possible energy states. The magnetic moment, like a compass needle, “wants” to align with the field, so that is the lower-energy state. Changing field, changing frequency. FIGURE 28.25 Energy levels for a proton in a magnetic field. align with a on. This isn’t ntum physics There are only orientations— Quantum mechanics limits the proton to two possible energies . . . Energy E2 5 1mB field e the field . . . which correspond to two possible orientations, aligned with or opposite the magnetic field. r B 0 E1 5 2mB µproton = 1.41× 10 −26 J/T If you increase the field from 1.0 T to 2.0 T, how does this change the frequency of the rf (radiofrequency) wave necessary to cause a spin flip? Quantum Weirdness. Quantum Weirdness: Non-locality Two places at one time Which slit did the electron go through? Where is the electron? From Chapter 12: vrms = 3kBT m Assume 85Rb m = 85 u Quantum Weirdness: Superposition Many things in the same place Quantum Weirdness: Mixed States Alive and dead cats Schrödinger’s Cat First, Back to the Rainbow Primary Colors Red Green Blue Complementary Colors Cyan Not Red Magenta Yellow Not Green Not Blue Fluorescence A range of wavelengths can excite electrons to the upper band. The electrons fall to the lower edge of the upper band. The electrons then jump to the lower band, emitting photons. Would you expect the absorbed or the emitted light to have a longer wavelength? Relative intensity Absorption band Emission band 0 300 400 500 600 Wavelength (nm)






