Test Protocols VIDAS® / mini VIDAS® Units VIDAS
advertisement

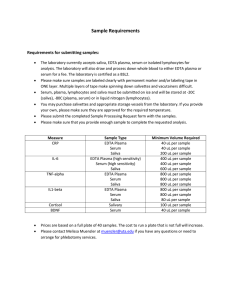
Test Protocols VIDAS® / mini VIDAS® Units VIDAS® / mini VIDAS® Compatible assays 01-13 / 9300651/008/ KR/A / This document is not legally binding. bioMérieux reserves the right to modify specifications without notice / BIOMERIEUX, the blue logo, VIDAS and D-Dimer Exclusion are used, pending and/or registered trademarks belonging to bioMérieux S.A. or one of its subsidiaries / bioMérieux SA RCS Lyon 673 620 399 / Photos: N. Bouchut, bioMérieux / Printed in France / THERA Conseil / RCS Lyon B 398 160 242 VIDAS test TSH T4 TSH3 FT3 FT4N CORS ATPO ATG T3 CKMB TNIU PBNP PBNP2 PCT MYO DEX2 PC HCG FER Ig E AFP STA ST STO (1) SE1 - SE2 - SM6 - SD1 SD2 - SW21 - SW6 - SG6 SG3 - ST9 - ST3 - ST6 - SK82 SI6 - SF85 - SF4 - SF3 SF14 - SF1 - SF2 - E2II TEST PRL LH FSH PRG CEAS 199 125 TPSA FPSA 153 CDAB HIV5 HIV6 Only parameters for which results can be expressed in different units are given in this table. Sample volume 200 µl 200 µl 200 µl 100 µl 100 µl 100 µl 250 µl 200 µl 150 µl 150 µl 200 µl 100 µl 100 µl 100 µl 200 µl 200 µl 200 µl 2 LH 200 µl 200 µl 200 µl 100 µl 200 VIDAS test Sample volume P24 P24 confirmation test MPG VZG MSG RBM TXC TXGA (0) TXG TXM RBG B2M LYT CMVG CMVU HPY CMVM HBCM II HBS HBS confirmation test HBST HBCT II HAVT HBE HBL HBL confirmation test HBET HAVM LYM LYG LYGS VCAG VCAM EBNA 100 µl 300 µl * 200 µl 200 µl 200 µl 200 µl * 100 µl 100 µl 125 µl 2 x 100 µl 100 µl µl 100 Parameter Proposed Code VIDAS Unit E2II FT4 T4 FT3 2 x 100 µl 100 µl 100 µl 100 µl 150 µl 150 µl * 150 µl 150 µl 150 µl 150 µl 150 µl T3 150 µl * 150 µl * 150 µl * 100 µl PRL 100 µl FER CORS B2M 100 µl 100 µl AFP TNIU PBNP PBNP2 PCT * = After sample processing 0 = After dilution to obtain 15 IU/ml 1 = These tests are performed with VIDAS Stallergy. pmol/l nmol/l pg/ml pg/ml ng/dl ng/100 ml pmol/l µg/l µg/100 ml µg/dl nmol/l pg/ml ng/l pmol/l ng/ml µg/l µg/100 ml µg/dl nmol/l µg/l ng/ml nmol/l µg/dl µg/l ng/ml µUI/ml µIU/ml ng/ml 1 IRP ng/ml 3 IS µg/ml UI/ml IU/ml mg/l µg/l ng/ml µg/ml IU/ml UI/ml ng/ml µg/l pmol/l pg/ml µg/l ng/ml Conversion Factors pg/ml x 3.67 pmol/l Parameter Proposed Code VIDAS Unit MYO pmol/l x 0.272 pg/ml pmol/l x 0.777 pg/ml pg/ml x 1.29 pmol/l nmol/l x 0.777 µg/l µg/l x 1.29 nmol/l CEAS FSH LH HAVT HCG pmol/l x 0.651 pg/ml pg/ml x 1.54 pmol/l nmol/l x 0.651 µg/l µg/l x 1.54 nmol/l PRG TES IGE nmol/l x 0.362 ng/ml ng/ml x 2.76 nmol/l ST TSH 1 ng 1st IRP = 32 µ UI 1 ng 3rd IS = 21 µ UI 1 ng/ml 3rd IS = 1.524 ng/ml 1st IRP TSH3 HBST mg/l x 14 UI/ml UI /ml x 0.071 mg/l P24 CMVG ng/ml x 0.826 UI/ml UI /ml x 1.21 ng/ml RBG TXG PC DEX2 µg/l ng/ml ng/ml mUI/ml UI/l mIU/ml IU/l mUI/ml UI/l mIU/ml IU/l kUI/l kIU/l mUI/ml nmol/l ng/ml nmol/l ng/ml UI/ml kIU/l IU/ml kUI/l kIU/l kUI/l mUI/l µIU/ml mIU/l µUI/ml µIU/ml µUI/ml mIU/ml mUI/ml pg/ml Ag P24 pg/ml HIV Ag AU/ml UA/ml UI/ml IU/ml IU/ml UI/ml UI/ml IU/ml % µg/ml ng/ml = Default unit FEU = Fibrinogen Equivalent Unit Conversion Factors ng/ml x 15.43 mUI/ml nmol/l x 0.3145 ng/ml ng/ml x 3.1796 nmol/l nmol/l x 0.288 ng/ml ng/ml x 3.47 nmol/l 1pg/ml of P24 = 3.65 pg/ml of HIV Ag (FEU) (FEU) VIDAS or mini VIDAS : Parameters Allergy Reproduction Fertility Tumor Markers ImmunoHemostasis Cardiovascular Others Measurement Range or Interpretation Code Sample Volume TSH 30400 TSH 200 µl S1 S1 C1 14 days no Serum, Plasma (Hep., Beads, Gel) 40 0.05 - 60 µIU/ml TSH3 30441 TSH3 200 µl S1 S1 S2 S2 C1 C2 14 days no Serum, Plasma (Hep., Silicone, Gel) 80 0.005 - 100 µIU/ml FT4 30459 FT4N 100µl S1 S1 C1 14 days no serum, plasma,(Hep.,silicon, gel) 40 1-100 pmol/ml FT3 30402 FT3 200 µl S1 S1 S1 C1 14 days no Serum, Plasma (Hep.) 40 0.7 - 45 pmol/l T4 30404 T4 200 µl S1 S1 S1 C1 14 days no Serum, Plasma (Hep.) 40 6 - 320 nmol/l T3 30403 T3 100 µl S1 S1 S1 C1 14 days no Serum, Plasma (Hep.) 40 Anti-TPO 30461 ATPO 100µl S1 S1 C1 28 days no serum, plasma,(Hep. EDTA, silicon, gel) Anti-Tg 30462 ATG 100µl S1 S1 C1 28 days no Total IgE 30419 IgE 100 µl S1 S1 C1 14 days Stallertest 30800 STA 200 µl S1 S1 C1 C2 Stallergy(6) 30801 ST 200 µl Stallertroph(5) 30830 STO HCG 30405 LH Parameter Test Frequency Sample Pre-Treatment Test time Reference* Range Thyroid Calibration-Control ® Test validated using (minutes) Range Parameter Reference* Code Calibration-Control Sample Volume Test Frequency Sample Pre-Treatment Test validated using Measurement Range or Interpretation Test time (minutes) TOXO IgG II 30210 TXG 100 µl S1 S1 C1 C2 14 days no Serum(3), Plasma (EDTA, Hep.) 40 TOXO IgM 30202 TXM 100 µl S1 S1 C1 C2 14 days no Serum(3) 40 TOXO Competition 30211 TXC 125 µl S1 S1 C1 C2 14 days no Serum, Plasma (EDTA, Hep.) 40 TOXO IgG Avidity (4) 30222 TXGA 2 x 100 µl C1 C2 14 days no Serum(3), Plasma (EDTA, Hep., Cit) 40 RUB IgG II 30221 RBG 100 µl S1 S1 C1 C2 14 days no Serum, Plasma (EDTA, Hep.) 40 0.4 - 9 nmol/l RUB IgM 30214 R BM 100 µl S1 S1 C1 C2 14 days no Serum 60 25 0,8-1000 IU/ml CMV IgG 30204 CMVG 100 µl S1 S1 C1 C2 14 days no Serum 40 serum, plasma,(Hep. EDTA, silicon, gel) 25 6,4-800 IU/ml no Serum, Plasma (Hep., EDTA) 30 0.5 - 1,000 kIU/l CMV IgG Avidity 30203 CMVU 2 x 100 µl CH CL 14 days no Serum 40 14 days no Serum, Plasma (Hep.) 90 Qualitative Test: Negative Positive or Equivocal CMV IgM 30205 CMVM 100 µl S1 S1 C1 C2 14 days no Serum 60 S1 S1 C1 14 days no Serum, Plasma 90 0.35 - 35 kIU/l EBV VC A IgM 30237 VC AM 100 µl S1 S1 S1 C1 C2 28 days no Serum 40 200 µl S1 S1 C1 14 days no Serum 90 0.35 - 35 kIU/l EBV VC A /EA IgG 30236 VC AG 100 µl S1 S1 S1 C1 C2 28 days no Serum 40 HCG 100 µl S1 S1 C1 14 days no Serum, Plasma (Hep., EDTA) 30 2 - 1,500 mIU/ml 30406 LH 200 µl S1 S1 C1 14 days no Serum, Plasma (Hep.) 40 0.1 - 100 mIU/ml EBV EBNA IgG 30235 EBNA 100 µl S1 S1 S1 C1 C2 28 days no Serum 40 FSH 30407 FSH 200 µl S1 S1 C1 14 days no Serum, Plasma (Hep.) 40 0.1 - 110 mIU/ml Lyme IgG and IgM 30298 LY T 100 µl S1 S1 C1 C2 14 days no Serum 35 Prolactin 30410 PRL 200 µl S1 S1 C1 14 days no Serum, Plasma (Hep.) 40 0.5 - 200 ng/ml Lyme IgM 30319 LYM 100 µl S1 S1 C1 C2 28 days no Serum, Plasma (Hep.) 27 Estradiol II 30431 E2II 200 µl S1 S1 S1 C1 14 days no Serum, Plasma (Hep.) 60 9 - 3,000 pg/ml Lyme IgG 30320 LYG 100 µl S1 S1 C1 C2 28 days no Serum, Plasma (Hep.) 27 Progesterone 30409 PRG 200 µl S1 S1 S1 C1 14 days no Serum, Plasma (Hep., EDTA, Silicone, Gel) 45 0.25 - 80 ng/ml LYGS 100 µl S1 S1 C1 C2 28 days no CSF (cerebrospinal fluid) 27 Testosterone 30418 TES 200 µl S1 S1 C1 14 days no Serum, Plasma (Hep.) 60 0.1 - 13 ng/ml Measles IgG 30219 M SG 100 µl S1 S1 C1 C2 14 days no Serum 40 CEA S 30453 CEAS 200 µl S1 S1 C1 14 days no Serum, Plasma (Hep.) 60 0.5 - 200 ng/ml Mumps IgG 30218 M PG 100 µl S1 S1 C1 C2 14 days no Serum 40 TPSA 30428 TPSA 200 µl S1 S1 C1 14 days no Serum, Plasma (Hep., EDTA) 60 0.07 - 100 ng/ml FPSA 30440 FPSA 200 µl S1 S1 C1 14 days no Serum, Plasma (Hep., EDTA) 60 0.05 - 10 ng/ml Varicella-Zoster IgG 30217 VZG 100 µl S1 S1 C1 C2 14 days no Serum 40 AFP 30413 AFP 100 µl S1 S1 C1 14 days no Serum, Plasma (Hep., EDTA), Amniotic Fluid 30 0.5 - 400 IU/ml H. Pylori IgG 30192 HPY 100 µl S1 S1 C1 C2 14 days no Serum, Plasma (EDTA) 40 CA 125 IITM 30426 125 200 µl S1 S1 C1 14 days no Serum, Plasma (Hep, EDTA) 60 4 - 600 U/ml HBs Ag Ultra (1) 30315 150 µl S1 S1 C1 C2 14 days no Serum, Plasma (Hep.) CA 19-9TM 30427 199 200 µl S1 S1 C1 14 days no Serum, Plasma (Hep., EDTA) 60 3 - 500 U/ml CA 15-3® 30429 153 100 µl S1 S1 C1 14 days no Serum, Plasma (Hep. EDTA) 60 2 - 400 U/ml Anti-HBsT Quick HBc IgM II 30238 30439 HBS • HBL HBST HBCM 150 µl 100 µl S1 S1 C1 C2 S1 S1 C1 C2 14 days 14 days no no Serum, Plasma (Hep.) Serum, Plasma (EDTA, Hep., Cit.) 60 90 60 55 Anti-HBc Total II 30314 HBCT 150 µl S1 S1 S1 C1 C2 14 days no Serum, Plasma (EDTA, Hep., Cit.) 90 D-Dimer ExclusionTM II (8) 30455 DEX2 200 µl S1 S1 C1 C2 28 days no Plasma (Cit.) 20 45 - 10,000 ng/ml (FEU) Protein C 30115 PC 100 µl S1 S1 C1 14 days no Plasma (Cit.) 35 1 - 120 % HBe Ag 30305 HBE 150 µl S1 S1 C1 C2 14 days no Serum, Plasma (EDTA, Hep., Cit.) 90 CK-MB 30421 CKMB 250 µl S1 S1 C1 14 days no Serum, Plasma (Hep., EDTA) 30 0 - 300 ng/ml Anti-HBe 30305 HBET 150 µl S2 S2 C2 C3 14 days yes Serum, Plasma (EDTA, Hep., Cit.) 90 Troponin I Ultra 30448 TNIU 200 µl S1 S1 S2 S2 C1 C2 28 days no Serum, Plasma (Hep.) 20 0.01 - 30 µg/l HAV IgM 30307 HAVM 100 µl S1 S1 C1 C2 14 days no Serum, Plasma (EDTA, Hep.) 60 Myoglobin 30446 MYO 150 µl S1 S1 C1 14 days no Serum, Plasma (Hep.) 17 5 - 1,000 µg/l Anti-HAV Total HIV P24 II (1) 30312 30117 HAVT P24 150 µl 200 µl S1 S1 C1 C2 S1 S1 C1 C2 14 days 14 days no no Serum, Plasma (EDTA, Hep., Cit.) Serum, Plasma (EDTA, Hep., Ox., Thrombin) 90 90 NT-proBNP 30449 PBNP 200 µl S1 S1 S2 S2 C1 C2 28 days no Serum, Plasma (Hep.) 20 20 - 25,000 pg/ml HIV DUO Quick 30447 HIV6 200 µl S1 S1 C1 C2 C3 14 days no Serum, Plasma (EDTA, Hep.)(2) 80 NT-proBNP2 30458 PBNP2 200 µl S1 S1 S2 S2 C1 C2 28 days no Serum, Plasma (Hep.) 20 20 - 25,000 pg/ml HIV DUO Ultra 30443 HIV5 200 µl S1 S1 S2 S2 C1 C2 C3 14 days no Serum, Plasma (EDTA, Hep.)(2) 120 Qualitative test: Negative, Positive or Equivocal Qualitative test: Negative, Positive or Equivocal Qualitative test: Negative, Positive or Equivocal Qualitative test: Negative, Positive or Equivocal Qualitative test: Negative, Positive or Equivocal Qualitative test: Negative, Positive or Equivocal Qualitative test: Negative or Positive Index, no interpretation (7) Qualitative test: Negative, Positive or Equivocal Qualitative test: Negative, Positive or Equivocal Qualitative test: Negative, Positive or Equivocal Qualitative test: Negative, Positive or Equivocal Qualitative test: Negative or Positive 5 - 500 mIU/ml 0 - 200 PEIU/ml Qualitative test: Negative, Positive or Equivocal Qualitative test: Negative or Positive Qualitative test: Negative, Positive or Equivocal Qualitative test: Negative, Positive or Equivocal 0 - 400 mIU/ml 0 - 400 pg/ml of Ag P24 Qualitative test: Negative or Positive Ag and Ab qualitative test: Negative or Positive Ferritin 30411 FER 100 µl S1 S1 C1 14 days no Serum, Plasma (Hep., EDTA) 30 1.5 - 1,200 ng/ml Antigen detection C. difficile Toxin A/B 30118 CDAB 300 µl S1 S1 C1 C2 C3 14 days yes(2) Stool 75 Qualitative test: Negative, Positive or Equivocal Cortisol S 30451 CORS 100 µl S1 S1 S1 C1 14 days yes (urine) Serum, Plasma (Hep., EDTA), 24-hour Urine 40 2 - 650 ng/ml ß2 Microglobulin 30420 B2M 100 µl S1 S1 C1 C2 14 days yes (urine) Serum, Plasma (Hep., EDTA), Urine 40 0.007 - 4 mg/l Severe Bacterial Infections BRAHMS PCT 30450 PCT 200 µl S1 S1 S2 S2 C1 C2 28 days no Serum, Plasma (Hep.)(2) 20 0.05 - 200 ng/ml Some of these reagents have not yet obtained regulatory clearance in some countries. Please contact your local bioMérieux representative for further information and product availability. * Some references may vary according the country. Please contact your local bioMérieux representative for further information and product availability. Serology Hepatitis / AIDS (1) = Confirmation test available (2) = See package insert (3) = Inactivated or not 30 mins at 56°C (4) = Double strips (5) = Test performed with VIDAS Stallergy (7) = Intrathecal antibody production has (6) = Reagent strips for Stallergy tests (20 specific IgE available) to be calculated by customer (8) = Will progressively replace D-Dimer Exclusion™ (order reference 30442) • •• 0 - 300 IU/ml Qualitative test: Negative, Positive or Equivocal Qualitative test: Negative or Positive Avidity index (AI) An AI ≥ 0,3 is a strong indication of a primary infection dating back more than 4 months. An AI < than 0,3 does not enable a recent infection to be differentiated from a former infection. 0 - 400 IU/ml Qualitative test: Negative, Positive or Equivocal 0 - 400 AU/ml Avidity index (AI) An AI ≥ 0,8 is a strong indication of a primary infection dating back more than 3 months. An AI < than 0,2 is a strong indication of a primary infection dating back less than 3 months. An 0,2 < AI < 0,8 does not enable to distinguish a recent infection from a former infection. HBL = long protocol CHB = test for confirmation of equivocal and positive CHL results Cit. = Citrate Ox. = Oxalate Hep. = Heparin VIDAS • PARAMETERS ®