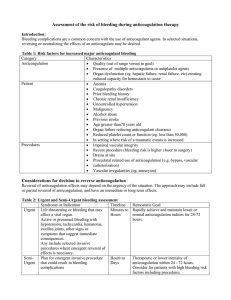
2016 ACC Expert Consensus Decision Pathway for Periprocedural
advertisement

EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 2016 ACC Expert Consensus Decision Pathway for Periprocedural Management of Anticoagulation A Report of the American College of Cardiology Clinical Expert Consensus Document Task Force PERIPROCEDURAL MANAGEMENT OF ANTICOAGULATION WRITING COMMITTEE John U. Doherty, MD, FACC, Chair Thomas L. Ortel, MD, PhD Ty J. Gluckman, MD, FACC, FAHA Sherry J. Saxonhouse, MD, FACC, FHRS William J. Hucker, MD, PhD Sarah A. Spinler, PharmD, FCCP, FAHA, James L. Januzzi Jr., MD, FACC FASHP, AACC, BCPS AQ-Cardiology TASK FORCE ON CLINICAL EXPERT CONSENSUS DOCUMENTS James L. Januzzi Jr., MD, FACC, Chair Luis C. Afonso, MBBS, FACC James K. Min, MD, FACC Anthony Bavry, MD, FACC Pamela B. Morris, MD, FACC Brendan Everett, MD, FACC John Puskas, MD, FACC Jonathan Halperin, MD, FACC Karol E. Watson, MD, FACC Adrian Hernandez, MD, FACC Oussama Wazni, MD, FACC Hani Jneid, MD, FACC Howard Weitz, MD, FACC Dharam Kumbhani, MD, SM, FACC Barbara S. Wiggins, PharmD, AACC Eva M. Lonn, MD, FACC This document was approved by the American College of Cardiology Board of Trustees in TBD 2016. The American College of Cardiology Foundation requests that this document be cited as follows: Doherty JU, Gluckman T, Hucker WJ, Januzzi JL, Ortel TL, Saxonhouse SJ, and Spinler SA. 2016 ACC Expert Consensus Decision Pathway for Periprocedural Management of Anticoagulation. J Am Coll Cardiol 2016; XX:XXX-XX. Copies: This document is available on the World Wide Web site of the American College of Cardiology (www.cardiosource.org). For copies of this document, please contact Elsevier Inc. Reprint Department, fax (212) 633-3820, e-mail reprints@elsevier.com. Permissions: Modification, alteration, enhancement, and/or distribution of this document are not permitted without the express permission of the American College of Cardiology. Requests may be completed online via the Elsevier site (http://www.elsevier.com/about/policies/author-agreement/obtaining-permission). © 2016 by the American College of Cardiology Foundation 1 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE CONTENTS ABSTRACT...................................................................................................................................................... 4 PREFACE ........................................................................................................................................................ 4 1. INTRODUCTION ...................................................................................................................................... 6 2. METHODS ............................................................................................................................................... 7 3. ASSUMPTIONS AND DEFINITIONS .......................................................................................................... 8 General Clinical Assumptions ................................................................................................................. 8 Definitions .............................................................................................................................................. 8 4. CENTRAL ILLUSTRATION ......................................................................................................................... 9 Figure 1. PMAC Decision Algorithm Outline .......................................................................................... 9 5. DESCRIPTION AND RATIONALE ............................................................................................................ 10 Periprocedural interruption of anticoagulant therapy ........................................................................ 10 Assessing procedural bleeding risk ...................................................................................................... 11 Assessing patient related bleeding risk ................................................................................................ 13 Table 1. Patient Bleed Risk Factors ..................................................................................................... 14 Table 2. Recommended Durations for Withholding DOACs Based on Procedural Bleeding Risk and Estimated CrCl When There are No Increased Patient Bleed Risk Factors ......................................... 18 Figure 2. Detailed Algorithm: Whether to Interrupt, and How to Interrupt for VKAs ....................... 19 Figure 3. Detailed Algorithm: Whether to Interrupt, and How to Interrupt for DOACs .................... 20 Parenteral bridging anticoagulation in the periprocedural setting...................................................... 21 Interruption and bridging for patients on DOACs ................................................................................ 21 Patients at low to moderate thrombotic risk ..................................................................................... 22 Patients at high thrombotic risk.......................................................................................................... 22 Patients at very high thrombotic risk .................................................................................................. 23 Specific recommendations regarding bridging .................................................................................... 24 Figure 4. Algorithm: Whether to Bridge and How to Bridge for DOACs and VKAs ............................ 26 Postprocedural reinitiation of anticoagulant therapy.......................................................................... 27 Restarting VKA therapy ........................................................................................................................ 28 Indications for postprocedural parenteral bridging and unique postprocedural indications.............. 29 Use of parenteral anticoagulation postprocedure in patients with high or very high thrombotic risk: Clinical factors and monitoring ............................................................................................................ 29 Reinitiation of DOAC therapy ............................................................................................................... 31 Postprocedural venous thromboembolism (VTE) prophylaxis............................................................. 35 Figure 5. Algorithm: How to Restart Anticoagulation ........................................................................ 38 6. DISCUSSION AND IMPLICATION OF PATHWAY .................................................................................... 39 APPENDIX A: AUTHOR RELATIONSHIPS WITH INDUSTRY AND OTHER ENTITIES ....................................... 40 APPENDIX B: PEER REVIEWER RELATIONSHIPS WITH INDUSTRY AND OTHER ENTITIES ........................... 41 2 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE APPENDIX C: COMMON PROCEDURES AND ASSOCIATED RISK .................................................................. 42 APPENDIX D: ABBREVIATIONS .................................................................................................................... 43 REFERENCES ................................................................................................................................................ 44 3 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 ABSTRACT 2 Periprocedural management of anticoagulation (PMAC) is a common clinical conundrum, involving 3 multiple members of the care team, cutting across many disciplines, and exhibiting high variability 4 among institutions and practices. Nowhere is this more evident than in the management of patients 5 with nonvalvular atrial fibrillation (NVAF). Standardized protocols, although deemed desirable, are 6 infrequently present. When implemented, however, such protocols improve patient outcomes. The 7 frequency of anticoagulant interruption is high, with an estimated 250,000 patients undergoing 8 temporary interruption (TI) annually in North America alone. Knowledge about risk of bleeding and short 9 term thrombotic risk resides in many specialties, further complicating the issue. Our goal in creating this 10 pathway is to help guide clinicians in the complex decision making in this area. In this document we 11 aimed to 1) Validate the appropriateness of the decision to chronically anticoagulated; 2) Guide 12 clinicians in the decision whether to interrupt anticoagulation (AC); 3) Direct them how to interrupt with 13 specific guidance for vitamin K antagonists (VKA) and direct-acting oral anticoagulants (DOAC); 4) 14 Evaluate whether to bridge with a parenteral agent periprocedurally; 5) Offer advice on how to bridge 15 and; and 6) Outline the process of restarting anticoagulation post-procedure. 16 PREFACE 17 The American College of Cardiology (ACC) develops a number of clinical policy documents to provide 18 members with guidance on clinical topics. While clinical practice guidelines remain the primary 19 mechanism for offering evidence based recommendations, such guidelines may contain gaps in how to 20 make clinical decisions, particularly when equipoise is present in a topic. Expert Consensus Documents 21 are intended to provide guidance for clinicians in areas where evidence may be limited, new and 22 evolving, or lack sufficient data to fully inform clinical decision making. 4 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 In an effort to increase the impact of ACC clinical policy on patient care, an ACC Presidential Task 2 Force was formed in 2014 to examine processes of ACC’s clinical documents. The main 3 recommendation of the Task Force was a new focus on concise decision pathways and/or key points of 4 care, instead of the traditional longer documents. The Task Force also established criteria for identifying 5 high-value clinical topics to be addressed, as well as an innovative approach to collecting stakeholder 6 input through a roundtable or think tank meeting (link to description on ACC.org – to be developed). To 7 complement the new focus on brief decision pathways and key points, Expert Consensus Documents 8 were rebranded Expert Consensus Decision Pathways (ECDP). 9 While Decision Pathways have a new format, they maintain the same goal of Expert Consensus 10 Documents to develop clinical policy based on expert opinion in areas which important clinical decisions 11 are not adequately addressed by the available existing trials. ECDPs are designed to complement the 12 guidelines and bridge the gaps in clinical guidance that remain. In some cases, topics covered by ECDPs 13 will be addressed subsequently by ACC/AHA guidelines as the evidence base evolves. The writing 14 groups are charged with developing algorithms that are more actionable and can be implemented into 15 tools or apps to accelerate the use of these documents at point of care. Decision Pathways are not 16 intended to provide a single correct answer, but to encourage clinicians to ask certain questions and 17 consider important factors as they come to their own decision on a treatment plan for their patients. 18 There may be multiple pathways that can be taken for treatment decisions and the goal is to help 19 clinicians make a more informed decision. 20 21 James L. Januzzi, JR, MD, FACC Chair, ACC Task Force on Clinical Expert Consensus Documents 22 5 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 1. INTRODUCTION 2 Atrial fibrillation (AF) is the most common sustained arrhythmia worldwide (1), with a prevalence that 3 substantially increases with age (2) and a lifetime risk of approximately 1 in 4 (3). This risk begins at age 4 40 and increases thereafter, such that at age 85, the prevalence of AF in an otherwise healthy 5 population approaches 18% (3). Antithrombotic therapy is recommended for most patients with AF to 6 reduce the risk of stroke and systemic embolism. By incorporating the known risk factors of heart 7 failure, hypertension, age, diabetes, stroke or TIA, vascular disease, and female gender into a scoring 8 system (the CHA2DS2-VASc score), strong preference is given to an oral anticoagulant (OAC) over 9 antiplatelet therapy in individuals with a score >2 (4-6). Although some controversy exists about the 10 relative importance of these risk factors (7,8), the CHA2DS2-VASc score better predicts ischemic events, 11 particularly among those with a lower-risk score (e.g., 0-1) in those judged with the simpler CHADS2 12 score (7,9-12); accordingly, CHA2DS2-VASc score has become the preferred score in clinical decision- 13 making (4,5). 14 Temporary interruption (TI), the omission of one or more doses of an OAC in preparation for a 15 procedure, is frequently necessary (13-18), most often to mitigate bleeding risk with surgical or invasive 16 procedures. While several factors are taken into consideration when making the decision to interrupt 17 anticoagulation (e.g., bleeding risk of the procedure, thrombotic risk associated with anticoagulant 18 interruption, and/or bleeding risk specific to the patient), there exists wide variation in practice. 19 Accordingly, this workgroup was convened to synthesize available data related to periprocedural 20 management of anticoagulant therapy for patients with nonvalvular atrial fibrillation (NVAF) by 21 specifically addressing: 1) when (if appropriate) anticoagulant therapy should be interrupted, 2) whether 22 (if appropriate) anticoagulant bridging with a parenteral agent should be performed, and 3) when and 23 how anticoagulant therapy should be restarted for those who require TI. 24 6 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 2. METHODS 2 For this document, we have restricted our data review and commentary to patients maintained on 3 chronic anticoagulation for NVAF, defined as AF in the absence of rheumatic mitral stenosis, a 4 mechanical or bioprosthetic heart valve, or mitral valve repair (4). Although this is a generally accepted 5 definition, trials varied as to whether patients with more than mild mitral regurgitation were included 6 (19-22). We address anticoagulant management in the preprocedure and postprocedure settings and 7 identify populations in whom TI of anticoagulation is not required. Finally, while this document can be 8 used to guide decision making for those undergoing urgent or emergent surgery, its primary goal is to 9 help direct management in elective, planned procedures. Though TI may be necessary for those taking 10 anticoagulant therapy for other indications (such as prior deep venous thrombosis [DVT], pulmonary 11 embolism, or prior valve replacement surgery), our comments cannot be extrapolated to these 12 populations. 13 For all patients on anticoagulant therapy for stroke prophylaxis in NVAF scheduled for a 14 procedure, it is important to carefully review the medical history, medication list, and laboratory test 15 results to identify factors that may increase bleeding risk. Based on these findings and the type of 16 procedure to be performed, the risks and benefits of TI should be discussed with, understood, and 17 agreed to by the patient. A collaborative discussion between the patient’s anticoagulation management 18 team and the practitioner performing the procedure or surgery should then follow. Before undertaking 19 the procedure, it is important to clearly document the anticoagulant management plan in the patient’s 20 medical record in order to minimize treatment errors. 21 22 7 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 3. ASSUMPTIONS AND DEFINITIONS 2 To limit inconsistencies in interpretation, specific assumptions were considered by the writing group in 3 development of the decision pathway. 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 General Clinical Assumptions 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 Definitions 1. This algorithm is only for patients with NVAF. 2. This algorithm assumes that the patient has a clinical indication for anticoagulation therapy, and is on the proper dose of anticoagulation therapy. 3. The algorithm assumes the patient is not taking concomitant antiplatelet agents, or if they are, that bleeding risk estimates may vary. 4. This algorithm is for elective planned procedures, not those occurring urgently or emergently. The section addressing postprocedural anticoagulant management, however, may still be relevant and should be considered. 5. In this document, the recommendations about withholding and resuming VKA therapy refer specifically to warfarin, which is the most common VKA in the United States. If outside the United States, check the pharmacokinetics of the VKA and adjust accordingly. Definitions of terms used throughout the indication set are listed here. Bridging: The process by which an OAC is discontinued and replaced by an injectable anticoagulant before, following or both before and following an invasive procedure Temporary Interruption: The process by which an anticoagulant is stopped for one or more doses resulting in full or partial dissipation of anticoagulant effect prior to the procedure Nonvalvular AF: AF in the absence of rheumatic mitral stenosis, a mechanical or bioprosthetic heart valve, or mitral valve repair Periprocedural: The period of time prior to, during and shortly following an invasive procedure Thromboembolic event (TE): Includes ischemic stroke, TIA or systemic embolism 8 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 4. CENTRAL ILLUSTRATION Figure 1. PMAC Decision Algorithm Outline 9 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 5. DESCRIPTION AND RATIONALE 2 3 4 Periprocedural interruption of anticoagulant therapy 5 assumption that 1) the patient has an appropriate clinical indication for the anticoagulant, 2) the 6 anticoagulant is dosed according to the product’s prescribing information, and 3) the patient is not 7 actively bleeding. 8 9 Implicit in any algorithm guiding periprocedural interruption of anticoagulant therapy in NVAF is the Current ACC/American Heart Association (AHA)/Heart Rhythm Society (HRS) and European Society of Cardiology (ESC) guidelines (4,5) recommend use of an OAC in those with NVAF and a 10 CHA2DS2-VASc score >2 (ACC/AHA/HRS—Class of Recommendation [COR] I, Level of Evidence [LOE] A for 11 use of adjusted-dose warfarin, a vitamin K antagonist [VKA] and COR I, LOE B for a direct oral 12 anticoagulant [DOAC]; ESC—COR I, LOE A for a VKA or DOAC). Differences exist, however, between the 13 guidelines as to whether an OAC should be used in those with NVAF and a CHA2DS2-VASc score of 1 14 (ACC/AHA/HRS—COR IIb, LOE C; ESC—COR IIa, LOE A). 15 In a recent retrospective review evaluating 140,420 patients with AF in the Swedish nationwide 16 health registries (6), the ischemic stroke rate in those with a CHA2DS2-VASc score of 1 was noted to be 17 lower than previously estimated (0.1-0.2% for women and 0.5-0.7% for men). In addition, a 18 retrospective cohort of Taiwanese patients demonstrated that age of 65-74 years was a more powerful 19 predictor of stroke in both men and women compared to other factors in the CHA2DS2-VASc score (23). 20 As such, it comes as no surprise how difficult it can be to settle on a single risk-benefit ratio for 21 anticoagulation in all populations. 22 Ultimately before one can determine whether TI is required for a given procedure, it is 23 important to first understand 1) the propensity for bleeding with the procedure, 2) the clinical impact of 24 bleeding should it occur, and 3) whether patient factors are present that impart increased bleeding risk. 10 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 2 3 4 Assessing procedural bleeding risk 5 to studies evaluating procedural risk; more commonly, such bleeding definitions are used to assess 6 bleeding severity in the context of clinical trials. Most data regarding prediction of risk for bleeding from 7 procedures come from small, observational studies and/or case series involving selected procedures. As 8 such, most guideline recommendations on this topic are based on expert consensus (14). Although standardized definitions for bleeding do exist (24,25), they have not been consistently applied 9 Just as important as the prevalence of bleeding, one must also consider its consequences. For 10 instance, even small amounts of bleeding in association with neuraxial anesthesia or after cardiac, 11 intraocular, intracranial, or spinal surgery may result in significant morbidity or mortality (26). As such, 12 procedures with low rates of bleeding, but significant associated sequelae should be categorized as high 13 risk. 14 A number of professional societies have published consensus documents, classifying their most 15 commonly performed procedures by bleeding risk and providing guidance as to how anticoagulant 16 therapy should be managed periprocedurally (27-37). Although some of these documents give guidance 17 for patients without AF, estimates of bleeding risk by procedure remains relevant. In these documents, 18 procedures have generally been categorized as high or low bleeding risk, with less common inclusion of 19 an intermediate bleeding risk category. Unfortunately, there are a number of procedures where 20 disagreement exists about how bleeding risk is categorized (e.g., hip/knee replacement, prostate biopsy, 21 endoscopic fine needle aspiration, hysterectomy) (38-42). In addition, the bleeding risk for many 22 procedures remains uncategorized. 23 24 For some procedures, uninterrupted oral anticoagulation with a VKA carries a lower bleeding risk compared to TI with bridging. This was observed in the BRUISE CONTROL (Bridge or Continue 11 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 Coumadin for Device Surgery Randomized Controlled) trial, where maintenance of therapeutic 2 anticoagulation with a VKA (goal INR of <3 on the day of the procedure) was associated with significantly 3 less bleeding compared to TI and bridging with heparin among those undergoing implantation of 4 pacemakers or ICDs (odds ratio [OR] of 0.19; p < 0.001) (43). Similar results were noted in the COMPARE 5 (Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation [AF] Patients Undergoing 6 Catheter Ablation) trial, where uninterrupted anticoagulation with a VKA (goal INR of 2-3) was 7 associated with lower rates of minor bleeding (p<0.001) and thromboembolic events (p < 0.001) 8 compared to TI and bridging with low molecular weight heparin (LMWH) in those undergoing catheter 9 ablation of AF (44). Based on the design of these two studies, however, relative bleeding risk in those 10 treated with an uninterrupted VKA versus TI without bridging and reinitiation of a VKA post procedure is 11 unknown. 12 Prospective data about the safety and efficacy of uninterrupted anticoagulation with the DOACs 13 are even sparser. Among patients undergoing catheter ablation of AF in the small VENTURE-AF (Active- 14 Controlled Multi-center Study with Blind-adjudication Designed to Evaluate the Safety of Uninterrupted 15 Rivaroxaban and Uninterrupted Vitamin K Antagonists in Subjects Undergoing Catheter Ablation for 16 Non-valvular Atrial Fibrillation) trial, patients maintained on either uninterrupted rivaroxaban or a VKA 17 had low rates of major bleeding (0.4%) and thromboembolic events (0.8%) (45). While other trials 18 evaluating this approach are underway, it is reasonable to also consider time-limited interruption of 19 anticoagulation without bridging in this patient population (46,47). 20 In conjunction with input from multiple professional societies, we classified the most commonly 21 performed procedures into 4 groups—those that are associated with 1) no clinically important bleeding 22 risk, 2) low procedural bleeding risk, 3) uncertain procedural bleeding risk, or 4) intermediate/high 23 procedural bleeding risk (Appendix C). Because the complexity of a given procedure may vary (for 24 instance, not all shoulder surgeries carry the same bleeding risk), an important caveat to this 12 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 categorization is acknowledgement that the proceduralist’s opinion of bleeding risk may vary from that 2 proposed in this document. 3 Assessing patient related bleeding risk (see Table 1) 4 Beyond the bleeding risks inherent to a given procedure, it is important to also assess patient-related 5 factors that may impart increased bleeding risk. These include a history of prior bleeding events (with 6 more weight placed on events within the last 3 months), bleeding complications associated with a 7 similar procedure in the past, qualitative or quantitative abnormalities of platelet function (for instance, 8 uremia) (48), concomitant use of antiplatelet therapy (or other medications associated with platelet 9 dysfunction), or for those taking a VKA, an INR in the supratherapeutic range (49-52). If possible, 10 providers should always delay the scheduled procedure to address patient-related factors that can be 11 corrected. Traditionally, the patient characteristics associated with increased bleed risk listed in Table 1 12 have been considered important. 13 Several risk scores have been proposed to generically evaluate bleeding risk in patients with AF 14 (49,50,52). The most widely used among these is the HAS-BLED score (9,52) which incorporates 15 hypertension, renal or hepatic impairment, prior stroke, TIA or systemic embolism, history of a major 16 bleed, a labile international normalized ratio (INR), and age greater than 65 years. While the HAS-BLED 17 score has been shown to have predictive value in the periprocedural setting (53), it is not commonly 18 used for this purpose. Instead, specific cut points for rates of major bleeding have been recommended 19 to differentiate those at high vs. low bleeding risk. In one review, procedures were considered high risk 20 if the two-day rate of major bleeding was 2-4% and low risk if the rate was 0-2% (38). In another, high 21 vs. low risk was defined by procedural rates of major bleeding >1.5% vs. <1.5%, respectively (39) . 22 13 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 2 Table 1. Patient Bleed Risk Factors Patient Bleed Risk Factors Hypertension * Abnormal renal/liver function * Stroke * Bleeding history or predisposition * Labile international normalized ratio (VKA) * Elderly (> 65 years) * Antiplatelet drugs/alcohol concomitantly * Prior bleed event within 3 months (including hemorrhagic stroke) ** Quantitative or qualitative platelet abnormality including aspirin use ** INR above the therapeutic range at the time of the procedure (VKA) ** Bleed history from previous bridging ** *Included in HAS-BLED(52) **Included in the periprocedural management algorithm 3 4 For patients taking a VKA: 5 Warfarin is the most commonly prescribed VKA worldwide. It inhibits the synthesis of vitamin-K 6 dependent clotting factors II, VII, IX, and X, as well as, the anticoagulant proteins C and S. It has a half- 7 life of approximately 36 to 42 hours and as such, requires advanced planning if TI is required. For 8 patients on warfarin, we propose the following approach periprocedurally (Figure 2). 9 10 11 Guidance Statement for determining whether a VKA should be interrupted periprocedurally: 1. Do not interrupt therapy with a VKA in: 12 13 Patients undergoing procedures with 1) no clinically important or low bleeding risk and 2) absence of patient-related factors that increase the risk of bleeding 2. Interrupt therapy with a VKA in: 14 Patients undergoing procedures with intermediate or high bleeding risk or 15 Patients undergoing procedures with uncertain bleeding risk and presence of patient- 16 related factors that increase the risk of bleeding 14 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 2 3 3. Consider interrupting a VKA based on both clinical judgment and consultation with the proceduralist in: 4 5 Patients undergoing procedures with 1) no clinically important or low bleeding risk and 2) presence of patient-related factors that increase the risk of bleeding or 6 Patients undergoing procedures with 1) uncertain bleeding risk and 2) absence of patient-related factors that increase the risk of bleeding 7 For all patients on a VKA, an INR level should be measured at least 1 week prior to the 8 procedure. This is done in individuals not requiring TI so that those with an INR >3.0 may be identified. 9 This is also done in individuals requiring TI to determine the number of days that the VKA should be 10 stopped prior to the procedure (Figure 2) 11 12 13 Guidance Statement as to how a VKA should be interrupted periprocedurally: 14 1. The VKA should be discontinued: For 3-4 days prior to the procedure in those with an INR <2.0, where a normal INR is 15 desired or for a shorter period of time if an elevated but subtherapeutic INR is 16 acceptable. 17 At least 5 days prior to the procedure in those with INR between 2.0 and 3.0. The exact 18 duration to withhold the VKA depends on the current INR, the time to the scheduled 19 procedure, and the desired INR for the procedure. The INR should be rechecked within 20 24 hours prior to the procedure. 21 At least 5 days prior to the procedure in those with an INR >3.0. The exact duration to 22 withhold the VKA depends on the current INR, the time to the scheduled procedure, 23 and the desired INR for the procedure. The INR should be rechecked within 24 hours 24 before the procedure. 25 15 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 2 3 For patients taking a DOAC: 4 apixaban, 2) dabigatran, 3) edoxaban, and 4) rivaroxaban. In spite of similar short times to peak activity, 5 fewer drug-drug interactions compared to VKAs, and the lack of need for routine monitoring, these 6 agents have distinctly different pharmacokinetics including their dosing frequency, dependence on renal 7 excretion, and criteria for dose adjustment (33). Their relatively short half-lives should reduce the 8 duration (compared to a VKA) for which preprocedural anticoagulation is withheld when TI is required. Four DOACs are currently approved to reduce the risk of stroke or systemic embolism in NVAF: 1) 9 It is important to bear in mind the pharmacokinetics of DOACs. Due to variation between the 10 peak and trough drug levels during the dosing interval with regular once a day or twice daily dosing, a 11 procedure performed at the trough level of a DOAC may allow a DOAC to be restarted the evening of or 12 the day after the procedure with only one or in some cases no dose(s) of the drug missed. 13 Since the DOACs became clinically available, one persistent concern for their use has been the 14 lack of a specific reversal agent in the case of major bleeding complications. This is particularly germane 15 in the periprocedural setting and in those that require repeat procedures. Recently, significant progress 16 has been made in this area with the approval of the monoclonal antibody fragment idarucizumab for the 17 reversal of dabigatran (54). Similar trials are in progress with 2 other novel agents, andexanet alfa and 18 ciraparantag, for reversal of the anticoagulant effects of LMWHs and factor Xa inhibitors (55,56). 19 For patients on a DOAC that require TI of anticoagulant therapy, it is imperative that renal 20 function be assessed. This should be done using the Cockcroft-Gault equation (with actual body weight) 21 to estimate creatinine clearance (CrCl). Importantly, insufficient data exists about the use of DOACs in 22 patients with a CrCl <15 mL/min. (Table 2). Nevertheless, such a situation may arise if the renal function 23 of a patient declines after a DOAC has been initiated. 24 25 The exact duration to withhold a DOAC depends upon the procedural bleeding risk, the specific agent, and the estimated CrCl. Because few data exist to provide guidance on periprocedural 16 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 management of DOACs in patients with stage V chronic kidney disease (CrCl <15 mL/min or on dialysis), 2 consideration should be given towards specific laboratory testing (e.g., dilute thrombin time for 3 dabigatran and agent-specific calibrated chromogenic anti-factor Xa activity for apixaban, edoxaban, and 4 rivaroxaban) in patients taking these agents. 5 In contrast to first assessing procedural bleeding risk in those on a VKA, we instead recommend 6 starting with assessment of patient-related factors in those taking a DOAC. This largely stems from a 7 paucity of data guiding which procedures can be safely performed in these patients without TI. In the 8 coming years, however, with greater anticipated types of procedures being performed on uninterrupted 9 DOAC therapy, we anticipate the need to refine this approach. 10 The recommended duration of TI for each DOAC relates to 1) the expected drug’s 11 clearance/metabolism, 2) the bleeding risk of the procedure, and 3) patient related factors that increase 12 bleeding risk. In patients with higher bleeding risk, electively scheduled procedures should be delayed, 13 if possible, to correct patient factors that potentiate bleeding risk. If the procedure cannot be delayed 14 or patient-related factors are not correctable, the DOAC should be interrupted as dictated by clinical 15 judgment. Although this document concerns itself with elective, planned procedures the use of a 16 reversal agent could be considered in patients undergoing an urgent/emergent procedure associated 17 with higher bleeding risk, requiring normal hemostasis and for which the procedure could not be 18 delayed for at least 8 hours (54). 19 Among those without patient-related factors that increase bleeding risk, it is important to next 20 assess procedural bleeding risk. In those undergoing procedures with no clinically important risk of 21 bleeding (Appendix C), the DOAC may only need to be held for a single dose. Alternatively, the 22 procedure could be performed without TI, but timed to coincide with the predicted nadir of the DOAC’s 23 drug level. Procedures routinely performed with a predictably low risk of bleeding (e.g., cataract 24 surgery) are arguably best performed with no or limited interruption but experience with this approach 17 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 using DOACs is limited. For those undergoing procedures with low, intermediate, high, or uncertain 2 bleeding risk, we propose the approach in Figure 3. 3 4 5 Guidance Statement for interruption of a DOAC periprocedurally: 1. Interrupt therapy for low bleeding risk procedures in: 6 7 Patients treated with any of the approved DOACs for a duration based on the estimated CrCl (Table 2). 8 2. Interrupt therapy for intermediate, high, or uncertain bleeding risk procedures in: 9 Patients treated with dabigatran for a duration based on the estimated CrCl (Table 2). 10 Patients treated with apixaban, edoxaban, and rivaroxaban for >48 hours. 11 12 13 Table 2. Recommended Durations for Withholding DOACs Based on Procedural Bleeding Risk and Estimated CrCl When There are No Increased Patient Bleed Risk Factors Dabigatran Apixaban, Edoxaban, or Rivaroxaban CrCl, in mL/min Procedural Risk ≥80 14 15 50-79 30-49 CrCl, in mL/min 15-29 <15 Low ≥24 hours ≥36 hours ≥48 hours ≥72 hours No data. Consider measuring dTT and/or withholding ≥96 hours Uncertain, Intermediate or High ≥48 hours ≥72 hours ≥96 hours ≥120 hours No data. Consider measuring dTT ≥30 15-29 <15 ≥24 hours ≥36 hours No data. Consider measuring agentspecific anti Xa level and/or withholding ≥48 hours ≥48 hours No data. Consider measuring agent-specific anti Xa level and/or withholding ≥72 hours The duration to withhold is based upon the estimated NOAC half-life (46,57). 18 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 Figure 2. Detailed Algorithm: Whether to Interrupt, and How to Interrupt for VKAs 2 19 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 Figure 3. Detailed Algorithm: Whether to Interrupt, and How to Interrupt for DOACs 2 20 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 Parenteral bridging anticoagulation in the periprocedural setting 2 Once the decision has been made to discontinue OAC therapy around the time of the procedure, the 3 next step is to develop a strategy that will 1) minimize the perioperative thrombotic risk while the OAC is 4 being withheld and 2) minimize the risk of perioperative bleeding. The DOACs have short half-lives that 5 obviate the need to administer an alternative anticoagulant during TI in the majority of situations. In 6 contrast, the anticoagulant effect of a VKA takes longer to dissipate once it is stopped and longer to 7 become therapeutic when restarted. Consequently, patients on a VKA who have a higher risk of 8 thromboembolic events may benefit from bridging using parenteral agents in the periprocedural setting. 9 Assessment of a patient’s thrombotic and bleeding risk is essential to determine the need for 10 bridging therapy while the VKA is being held. While the timing of interruption and the decision to bridge 11 with a parenteral anticoagulant is based on the patient’s risk of thromboembolism, there are no 12 validated assessment schemes to determine this risk. Extrapolating risk for a thrombotic event as a 13 function of the period of interruption based on the annual risk may be attractive but has not been 14 validated. Another option may be use of the CHA2DS2-VASc score to identify high-risk patients. As the 15 thrombotic risk increases, the need for bridging becomes more apparent, unless a compelling risk of 16 bleeding is present. 17 Interruption and bridging for patients on DOACs 18 Given the short-half lives of DOACS, bridging with a parenteral agent is rarely, if ever, needed prior to 19 procedures. Reinitiation of these agents after the procedure, however, may need to be delayed 20 depending upon 1) the need for additional procedures, 2) the risk of postprocedural bleeding, and 3) the 21 patient’s ability to tolerate oral medications. Therefore, a parenteral anticoagulant may be needed 22 either between procedures, or post-procedure when thrombotic risk remains high. These are very 23 specific scenarios which are uncommon in routine clinical practice. 21 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 2 3 4 Interruption and bridging for patients on VKA Patients at low to moderate thrombotic risk Long-term thrombotic risk in NVAF rises proportionally with the CHA2DS2-VASc score, especially 5 among patients with prior stroke or TIA (14,58,59). For patients with a CHA2DS2-VASc score of 3 or less 6 and no prior history of ischemic stroke or TIA, the risk for a thrombotic event is low (<5%/year). As such, 7 these patients may discontinue the VKA prior to the procedure as articulated, with resumption when it 8 is felt to be safe from a procedural bleeding risk standpoint as discussed below. Therefore, under most 9 circumstances no preprocedural or postprocedural parenteral anticoagulation is recommended. 10 11 12 13 Guidance Statement for determining appropriateness for bridging in those on a VKA at low to moderate risk for thromboembolism: 1. For patients at low to moderate risk for thromboembolism (<5%/year), with a CHA2DS2-VASc 14 score of 3 or less and no prior history of ischemic stroke or TIA, discontinue the VKA prior to 15 the procedure and resume as discussed below. 16 17 Patients at high thrombotic risk For individuals at high risk for thrombotic events with a CHA2DS2-VASc score of 4 to 5 or prior history 18 of ischemic stroke or TIA, it is important to determine the patient’s bleeding risk as this dictates the 19 appropriateness of bridging therapy. In this group, individuals with higher bleeding risk should have 20 their VKA withheld without parenteral bridging. For those without significant bleeding risk undergoing TI 21 of their VKA, bridging 1) should likely be performed in those with prior stroke or TIA and 2) should likely 22 be withheld in those without prior stroke or TIA. 23 24 Guidance Statement for determining appropriateness for bridging in those on a VKA at high risk for thromboembolism 22 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 1. For patients at high risk of stroke or systemic embolism (SE) with a CHA2DS2-VASc score of 4 to 2 5 or history of prior ischemic stroke or TIA, the patient’s bleeding risk is important to 3 determine the appropriateness of bridging therapy. 4 5 6 7 8 2. For patients at high thrombotic risk with increased risk of bleeding, interruption of the VKA without bridging is recommended. 3. For patients at high risk of stroke or SE with no significant bleeding risk: a. Consider use of a parenteral anticoagulant for periprocedural bridging if they have had a prior stroke or TIA (Use Clinical Judgement, likely bridge) 9 b. Probably do not use a parenteral anticoagulant for periprocedural bridging if they 10 have not had a prior stroke or TIA (Use Clinical judgement, likely do not bridge) 11 12 Patients at very high thrombotic risk Patients at very high thrombotic risk for stroke or SE, such as those with a CHA2DS2-VASc of 6 or 13 greater or with a recent thrombotic event (<3 months), have a risk of thromboembolic complications 14 that should generally be managed with bridging. Importantly, for those with a recent thrombotic event 15 (<3 months), the procedure should ideally be delayed, if possible, to move beyond this timeframe. For 16 those with a recent intracranial hemorrhage (<3 months), the procedure should be performed either 17 with no bridging or post procedural bridging only. Clinical judgment should be used to guide bridging in 18 those at high bleeding risk, but without recent intracranial hemorrhage. 19 20 21 Guidance Statement for determining appropriateness for bridging in those on a VKA at very high risk for thromboembolism: 1. For patients at very high risk of thromboembolism with a CHA2DS2-VASc score of 6 or greater 22 or recent ischemic stroke or TIA (<3 months), parenteral bridging anticoagulation should be 23 performed. 23 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 2 Specific recommendations regarding bridging Use of bridging with a parenteral anticoagulant is common, yet the bulk of current evidence suggests 3 that it is associated with an increased risk of both major adverse cardiovascular events and major 4 bleeding, without a significant decrease in thromboembolic events (17,18,42,60). In spite of this, 5 bridging may be appropriate in certain clinical scenarios, such as in patients at high and very high risk of 6 stroke or SE. Multiple prior observational studies have evaluated various parenteral agents, dosing 7 schemes, and timings for periprocedural parenteral anticoagulation, yet no single agent or dosing 8 regimen has been deemed to be superior (61-65). 9 Various parenteral anticoagulants may be used for bridging. Most commonly, unfractionated 10 heparin (UFH) or a low molecular weight heparin (LMWH) is used; however, argatroban may be used for 11 patients with a history of heparin-induced thrombocytopenia. In those with NVAF, use of a LMWH has 12 been associated with decreased length of hospitalization with similar rates of thromboembolism and 13 bleeding rates compared to UFH (64). For those using a LMWH in the periprocedural setting, close 14 attention to renal function is necessary to ensure proper dosing. 15 The parenteral anticoagulant can be started 2-3 days after stopping the VKA, or when the INR is 16 <2.0. The decision to use UFH versus LMWH as the bridging agent depends upon 1) estimated renal 17 function (based on the CrCl), 2) the parenteral bridging setting (inpatient versus outpatient), 3) patient 18 comfort with self-injections, and 4) insurance coverage. If the CrCl is <30 mL/min, UFH is preferred over 19 LMWH; however, dosing guidance for LMWH is available for patients with a CrCl of 15-30 mL/min. 20 Therapeutic anticoagulation is recommended until the time of procedure. UFH and argatroban 21 (for patients with a past history of heparin-induced thrombocytopenia) may be discontinued as late as 4 22 hours prior to the procedure, with the option of assessing coagulation with the activated partial 23 thromboplastin time (aPTT). If a LMWH is used, it will need to be discontinued at least 8 hours prior to 24 the procedure, with the option, if necessary, of assessing residual anticoagulation by checking a LMWH- 25 specific anti-factor Xa level. 24 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 2 3 4 Guidance Statement for preprocedural management of parenteral bridging anticoagulation for those on a VKA: 1. While UFH or a LMWH is most commonly used for bridging, the parenteral direct thrombin 5 inhibitor argatroban may be used in those with a history of heparin-induced 6 thrombocytopenia. 7 8 9 10 11 12 2. Start parenteral anticoagulant therapy 2-3 days after stopping the VKA or when the INR is <2.0. 3. Discontinue UFH and argatroban four hours prior to the procedure; the residual anticoagulant effect may be measured by the aPTT. 4. Discontinue LMWH at least eight hours prior to the procedure; the residual anticoagulant effect may be measured by a LMWH-specific anti-factor Xa assay. 25 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 Figure 4. Algorithm: Whether to Bridge and How to Bridge for DOACs and VKAs 2 26 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 Postprocedural reinitiation of anticoagulant therapy 2 Restarting anticoagulation in the postprocedural setting may place the patient at significant risk for 3 bleeding. In patients managed with TI of anticoagulation, recent studies have documented an overall 4 bleeding risk of 2.2-2.3% without bridging, with even higher rates in patients bridged with parenteral 5 anticoagulation (18,42,61). Importantly, poastprocedural bleeding risk is dependent on 1) the timing of 6 anticoagulation reinitiation, 2) the type of procedure performed, and 3) intra-procedural findings, 7 changes to the planned procedure, or complications, and 4)the anticoagulant used. Together, these 8 factors determine the post-procedural risk for bleeding. Often, the post-procedural risk will reflect the 9 pre-procedural bleeding risk of the procedure (e.g., high bleeding risk, or low bleeding risk), however 10 details of the particular procedure that the patient underwent may shift that risk in one direction or the 11 other. 12 Postprocedural reinitiation of anticoagulation must first begin with a careful assessment of the 13 procedure site to determine adequacy of hemostasis. This necessitates a team-based approach 14 involving the primary managing service with the surgeon/proceduralist. It is also important to assess 15 consequences of bleeding. For instance, bleeding after spinal or intracranial procedures carries a 16 significantly higher risk of morbidity and mortality. Finally, one should assess patient characteristics that 17 increase bleeding risk. Any history of recent bleeding, qualitative or quantitative abnormalities in 18 platelets (including effects of antiplatelet medications), or abnormalities in coagulation studies should 19 influence when anticoagulation is resumed. 20 When considering resumption of anticoagulation after TI in patients with NVAF, it is important 21 to balance bleeding risk against thrombotic risk. In the BRIDGE (Bridging Anticoagulation in Patients who 22 Require Temporary Interruption of VKA Therapy for an Elective Invasive Procedure or Surgery) trial, the 23 overall rate of thromboembolism in patients with NVAF was 0.4%, with no difference noted between 24 bridged and non-bridged patients (42). Importantly, the average CHADS2 score in this trial was 2.3 in the 27 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 non-bridged group and 2.4 in the bridged group, with <15% of the total cohort having a CHADS2 score 2 4. Therefore, these results may not be as applicable to patients with higher thromboembolic risk. 3 In a separate, large observational registry of patients with AF, the overall rate of thrombotic 4 events was 0.6% in bridged and non-bridged patients managed with TI of OAC therapy (18). In contrast, 5 a higher rate of thromboembolism (2.3%) was observed in a smaller non-randomized study of patients 6 treated with bridging (61). Even with these differences, the takeaway is that the rate of periprocedural 7 thromboembolism is relatively low and as such, this risk must be weighed against the risk of bleeding. 8 9 Guidance statement for restarting anticoagulation postprocedure: 1. Ensure procedural site hemostasis. 10 11 12 13 2. Consider bleeding consequences, especially with high bleeding risk procedures such as cardiac surgery, intracranial, or spinal procedures. 3. Consider patient specific factors that may predispose to bleeding complications (i.e., bleeding diathesis, platelet dysfunction, antiplatelet medications). 14 Restarting VKA therapy 15 The timing of anticoagulant reinitiation should always be made as a team, involving the proceduralist 16 and the primary managing service. Once hemostasis is achieved and no obvious bleeding complications 17 are present, reinitiation of a VKA can usually occur in the first 24 hours following the procedure, typically 18 at the patient’s regular therapeutic dose (14,42). Early postprocedural initiation of a VKA will not 19 increase the early risk of bleeding because its anticoagulant effect typically begins 24-72 hours after 20 initiation of therapy. In general, the full therapeutic effect occurs 5-7 days after initiation, assuming the 21 INR was normal at the time of initiation. The anticoagulant effect of a VKA is closely related to hepatic 22 function, antibiotic use, nutritional status, and interaction with other medications, all of which can 23 change in the postprocedural setting. If this occurs, the VKA dosing may need to be adjusted. 28 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 In the setting of 1) any postprocedural bleeding complication, 2) a procedure at high risk for 2 bleeding, or 3) presence of patient specific factors that increase the risk of postprocedural bleeding, 3 delayed reinitiation of anticoagulation may be considered. 4 5 Guidance statement for the postprocedural timing of VKA reinitiation: 1. In most situations, a VKA can be restarted in the first 24 hours after the procedure at 6 the patient’s usual therapeutic dose. 7 Indications for postprocedural parenteral bridging and unique postprocedural indications 8 For many patients, periprocedural bridging is not necessary and may increase the risk of significant 9 bleeding complications. Thus, careful selection based on thrombotic and bleeding risks is essential to 10 determine the best strategy for most patients. Patients on a VKA who are at high or very high risk for 11 stroke or systemic thromboembolism may resume parenteral agents until the target INR is achieved. If 12 there is concern about the use of postprocedural parenteral bridging because of high bleeding risk, one 13 should consider reinitiation of the VKA without bridging. In selected circumstances, patients may have a 14 second procedure scheduled during the same period and will need to continue the parenteral 15 anticoagulant in between procedures without resuming their OAC. 16 17 Guidance Statement for consideration of postprocedural parenteral anticoagulation: 1. Postprocedural bridging with a parenteral agent can be used for patients with high and very 18 19 20 high stroke or SE risk. 2. VKA therapy should be resumed (in most cases at the patient’s usual therapeutic dose) without use of parenteral anticoagulation in cases associated with high risk for bleeding. 21 22 23 Use of parenteral anticoagulation postprocedure in patients with high or very high thrombotic risk: Clinical factors and monitoring Timing the initiation of postprocedural parenteral anticoagulation depends on the type of procedure 24 performed, as well as the extent of hemostasis (14). If parenteral anticoagulation is used after 25 procedures with low bleeding risk (Appendix C), we recommend its initiation within 24 hours after the 29 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 procedure, assuming hemostasis has been achieved and the postprocedural bleeding risk is still deemed 2 to be low. In contrast, we recommend delaying therapeutic parenteral anticoagulation, if possible, for 3 48-72 hours following procedures with high bleeding risk (Appendix C) (14,42). Earlier initiation of a 4 parenteral agent in this latter group is associated with an increased risk of major bleeding (14,61). 5 When bleeding risk is elevated but thrombotic risk is also considered to be high, individualized strategies 6 may be considered. Options to minimize bleeding risk include 1) initiation of UFH without a bolus dose, 7 2) dosing of UFH or LMWH at a lower dose (such as those used for DVT prophylaxis), or 3) initiation of a 8 VKA alone (14,63). 9 Frequent monitoring of coagulation is required during bridging anticoagulation. In addition to 10 monitoring the aPTT with UFH or argatroban, a chromogenic factor X assay may be assessed when 11 transitioning between argatroban and a VKA because argatroban elevates the INR (66). However the 12 PT/INR must also be routinely monitored during bridging when the VKA is restarted, as the risk of 13 bleeding increases as the INR enters the therapeutic range (67). In the BRIDGE trial, the median time to 14 a major bleed was 7.0 days, with the majority of these events occurring in patients randomized to 15 bridging anticoagulation (42). This suggests that the time of highest risk for bleeding is when the 16 therapeutic INR is nearly reached. 17 18 19 Guidance statement for the initiation of postprocedural therapeutic parenteral anticoagulation in patients with high or very high thrombotic risk: 1. Establish that hemostasis has been achieved, procedure specific bleeding complications 20 have been considered, patient specific bleeding factors have been evaluated, and both the 21 proceduralist and primary managing service are involved in the decision to restart 22 anticoagulation. 23 2. Following procedures at low postprocedural risk of bleeding, therapeutic parenteral 24 anticoagulation, if indicated, can be started in the first 24 hours after the procedure. 30 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 2 3. Following procedures at high postprocedural risk of bleeding, therapeutic parenteral anticoagulation should be delayed for 48-72 hours after the procedure. 3 4. Careful monitoring of the INR during bridging is required to mitigate bleeding risk. 4 5. LMWH or UFH should be discontinued when the INR is 2.0. This approach is modified if 5 argatroban is used (see text). 6 7 8 Reinitiation of DOAC therapy 9 important to consider the consequences of procedural site bleeding and patient-related factors that Similar to a VKA, reinitiation of a DOAC first requires hemostasis at the procedural site. Thereafter, it is 10 increase the likelihood of bleeding complications. Unlike therapy with a VKA, use of a DOAC will render 11 the patient therapeutically anticoagulated within hours after the first full DOAC dose. As such, the 12 timing of postprocedural DOAC reinitiation should be considered similarly to the timing of parenteral 13 anticoagulation discussed previously, and in most clinical situations, no parenteral agent is needed if 14 resumption of DOAC therapy is planned. Renal function must be carefully monitored in the 15 postprocedural setting, as renal impairment affects the dosing of all DOACs for NVAF. 16 17 18 Dabigatran There are several studies investigating TI of DOACs in the periprocedural setting. A substudy of the RE- 19 LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial analyzed 4,591 patients that had 20 OAC therapy interrupted for a procedure. Similar rates of major bleeding were observed with dabigatran 21 150 mg twice daily and VKA therapy (3.8% vs. 3.3%), with shorter interruption of anticoagulation needed 22 with dabigatran (13). In this study, dabigatran was restarted after the procedure once hemostasis was 23 achieved; the details of how dabigatran was resumed were not specified. A separate analysis of this 24 population found that the use of parenteral bridging anticoagulation together with dabigatran resulted 31 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 in >3 times more major bleeding (6.5% in bridged patients vs 1.8% in those not bridged) with no 2 statistical difference in thromboembolic events (60). 3 A predefined postprocedure management algorithm for dabigatran has been previously 4 reported (68). For low bleeding risk procedures (including use of neuraxial anesthesia), dabigatran was 5 resumed at a reduced dose of 75 mg on the night of the procedure (4 hours after neuraxial anesthesia), 6 with resumption of the full dose the following morning. In contrast, dabigatran was resumed at full dose 7 48-72 hours after the procedure for high bleeding risk procedures. Using this algorithm, the incidence of 8 major bleeding and thromboembolism was 1.8% and 0.2% respectively, both of which compare 9 favorably with outcomes from other studies of periprocedural VKA therapy. 10 11 12 Rivaroxaban Similar to dabigatran, a sub-study of the ROCKET-AF (Rivaroxaban Once Daily Oral Direct Factor Xa 13 Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial 14 Fibrillation) trial analyzed periprocedural outcomes after TI in 4,692 patients. Similar low rates of 15 thromboembolism were noted with rivaroxaban and VKA therapy during the at-risk period (0.3% versus 16 0.4%), with no difference in major bleeding (15). While this provides support that TI is relatively safe 17 with rivaroxaban, details about how it should be resumed postprocedure are not known. 18 A large registry of patients (n=2,179) treated with a DOAC (the majority of whom were on 19 rivaroxaban) reported that TI was frequent, with resumption of therapy most commonly occurring 1 day 20 after the procedure (17). This approach was associated with an 1.2% rate of major bleeding in the 30 21 days following the procedure, with 0%, 0.5%, and 8% rates of major bleeding following minimal (n=135), 22 minor (n=641), and major (n=87) procedures in the study. Following major procedures, 6 of the 7 major 23 bleeding events occurred in patients who received some form of bridging anticoagulation, and there was 24 no difference in rates of major cardiovascular events with bridging. It is important to note that a 25 majority (90%) of procedures in this study were considered minor in bleeding risk. Furthermore, most of 32 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 these procedures would qualify as low risk bleeding procedures by the classification outlined in this 2 document, underscoring the difficulty in comparing bleeding rates across studies. 3 Unlike with dabigatran, there are no data on the use of reduced doses of rivaroxaban beginning 4 on the evening after low bleeding risk procedures in the setting of NVAF. However, this strategy has 5 been used for DVT prophylaxis after orthopedic procedures associated with high bleeding risk. Pooled 6 analysis of several randomized trials found a trend towards more major bleeding with rivaroxaban 10 7 mg started 6-8 hours after the procedure when compared to LMWH, though the overall risk of major 8 bleeding was low with both approaches (69). 9 10 11 Apixaban In a pre-specified analysis from the ARISTOTLE (Apixaban for Reduction in Stroke and Other 12 Thromboembolic Events in Atrial Fibrillation) trial, apixaban (compared to VKA therapy) was associated 13 with similar rates of thromboembolism and major bleeding in the periprocedural period, establishing it 14 as a relatively safe option if TI is required (16). Despite the lack of an approved reversal agent for 15 apixaban, periprocedural bleeding outcomes were similar between apixaban and a VKA, with no 16 difference in the rate of thromboembolism, regardless of whether anticoagulation was stopped 17 periprocedurally. Notably, however, only 10% of procedures in this study were classified as “major”, 18 defined by the need for general anesthesia. 19 Similar to rivaroxaban, apixaban has been studied in the prophylaxis of DVT after orthopedic 20 surgery, with initiation of therapy 12-24 hours after surgery. While no significant increase in bleeding 21 has been observed when compared to LMWH in this population, no prophylactic dosing for 22 postprocedural thromboprophylaxis in NVAF has been published (46). 23 24 25 Edoxaban To date, there have been no published data regarding edoxaban in the periprocedural period; however, 26 an analysis of the ENGAGE-AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in 33 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial suggests that procedures performed on 2 edoxaban had similar outcomes compared to VKA therapy among those with or without TI (70). Similar 3 to other DOACs, manufacturer recommendations, as well as expert consensus suggest that after 4 complete hemostasis is achieved, it is reasonable to resume full dose edoxaban 6-8 hours after a 5 procedure. Following procedures with a high risk of bleeding, though, it is reasonable to delay 6 resumption of full dose edoxaban for 48-72 hours (46). 7 8 Guidance statement for restarting DOAC therapy postprocedure: 1. Establish that hemostasis has been achieved, procedure specific bleeding complications have 9 been considered, patient specific bleeding factors have been evaluated, and the proceduralist 10 11 and primary managing service has been involved in the decision to restart anticoagulation. 2. Following procedures with low postprocedural bleeding risk where TI is indicated, it is 12 reasonable to resume DOAC therapy at full dose on the day following the procedure; 13 administration of prophylactic dosing on the evening of the procedure could be considered. 14 3. 15 Following high postprocedural bleeding risk procedures, it is reasonable to wait 48-72 hours before resuming DOAC therapy at full dose if complete hemostasis has been achieved. 16 4. DOAC dosing should reflect postprocedural renal function. 17 5. Bridging therapeutic anticoagulation with a parenteral agent is generally not required. 18 19 Scenarios requiring special consideration for DOAC re-initiation 20 Prolonged period of inability to take oral medications following a procedure in patients taking a DOAC 21 A parenteral agent is not typically required when using a DOAC, however in patients unable to tolerate 22 oral medications for a prolonged period postprocedurally (e.g., postoperative ileus after abdominal 23 surgery), use of a parenteral agent may be necessary to manage TI of anticoagulation. In this situation, 24 we recommend that therapeutic anticoagulation with a parenteral agent begin within the first 24 hours 25 following a procedure with low bleeding risk and within 48-72 hours after a procedure with high 34 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 bleeding risk (46,57,71). In patients receiving or who have received procedural neuraxial anesthesia, 2 (see below), careful attention to the timing of catheter placement and withdrawal is necessary and we 3 refer the reader to the product-specific prescribing information and the American Society of Regional 4 Anesthesia and Pain Medicine Guidelines (35,72-75). 5 If a LMWH is used postprocedure, the chosen dose should reflect the patient’s renal function in 6 the postprocedural setting. When the patient can tolerate oral medications, the LMWH should be 7 discontinued and the DOAC can be resumed at the time that the next scheduled LMWH dose would 8 have been administered. If UFH is used, the DOAC can be started at the time that UFH is discontinued 9 (19-22). Of note, rivaroxaban must be taken with a meal to have full therapeutic effect; rivaroxaban and 10 apixaban may be crushed and administered with water or applesauce in a feeding tube (72,73). 11 Postprocedural venous thromboembolism (VTE) prophylaxis 12 Resumption of a DOAC or therapeutic parenteral anticoagulation for NVAF obviates the need for other 13 anticoagulants for VTE prophylaxis post-procedure. For procedures that result in immobility that require 14 VTE prophylaxis in patients at high risk of bleeding, nonpharmacologic measures, such as an intermittent 15 pneumatic compression device, may be used if appropriate. For some patients, there may be a need to 16 provide prophylactic doses of anticoagulation prior to the resumption of therapeutic anticoagulation. 17 During TI of DOAC therapy, it is reasonable in these cases to use prophylactic doses of LMWH or UFH 18 starting 6-8 hours following the procedure for VTE prophylaxis provided that adequate hemostasis has 19 been achieved (46). In the setting of NVAF, only dabigatran has been specifically tested at a prophylactic 20 dose after low risk bleeding procedures, with a low overall rate of major bleeding (1.8%) (68). Following 21 orthopedic surgery, apixaban 2.5 mg twice daily, edoxaban 15 mg or 30 mg daily, and dabigatran 150 mg 22 or 220 mg daily for VTE prophylaxis have similar rates of major or clinically relevant nonmajor bleeding 23 compared to prophylactic doses of enoxaparin, while rivaroxaban 10 mg daily has a higher rate of major 24 or clinically relevant nonmajor bleeding (76,77). Rivaroxaban 10 mg daily and apixaban 2.5 mg twice 35 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 daily demonstrated significantly more major or clinically relevant nonmajor bleeding than prophylactic 2 enoxaparin for VTE prophylaxis in medically ill patients (78). It is worth noting that postprocedural 3 prophylactic doses of UFH or LMWH as well as DOACs administered in doses lower than indicated for 4 NVAF may not fully protect against TE in AF patients. 5 6 Neuraxial anesthesia 7 Use of anticoagulants in the setting of neuraxial anesthesia raises the risk of a spinal or epidural 8 hematoma, which can have dire consequences. All currently available DOACs carry a black box warning 9 regarding their use in the setting of neuraxial anesthesia. The prescribing information for each oral 10 factor Xa inhibitor (apixaban, edoxaban, and rivaroxaban) provides specific guidance on the minimal 11 length of time after the last DOAC dose that an epidural catheter may be removed, as well as, the 12 minimal amount of time after catheter removal when the DOAC can be restarted. In the case of 13 dabigatran, the prescribing information does not provide specific timing recommendations for epidural 14 catheter removal or anticoagulation reinitiation. The American Society of Regional Anesthesia and Pain 15 Management has developed guidelines regarding the periprocedural management of antiplatelet and 16 anticoagulant medications around pain procedures. Their guidelines recommend discontinuing a DOAC 17 for 5 half lives prior to neuraxial anesthesia (4-5 days for dabigatran, 3-5 days for factor Xa inhibitors), 18 with reinitiation 24 hours postprocedure (35). Of note, this is significantly longer than that 19 recommended by the package inserts for the DOACs (72-75), and in fact one study restarted dabigatran 20 4 hours after epidural catheter removal after orthopedic procedures (68). 21 22 23 Restarting anticoagulation after a procedure with unknown bleeding risk The timing of restarting anticoagulation as discussed above is largely based on the bleeding risk of the 24 procedure performed. However, for procedures where the bleeding risk is unknown, precise guidance 25 regarding the timing of restarting anticoagulation is difficult. As previously noted, published rates of 36 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 periprocedural thromboembolism are low, yet the bleeding risk following these procedures is unknown. 2 Therefore, in the absence of evidence-based data, we recommend approaching reinitiation of 3 anticoagulation as was previously recommended for high bleeding risk procedures. This will delay the 4 reinitiation of anticoagulation after the procedure; however, it will not significantly increase the 5 thromboembolic risk for most patients. 6 7 8 Restarting DOAC therapy following a cardiac procedure If the patient is on a DOAC prior to a valve replacement or repair then that patient doesn’t meet the 9 criteria for having NVAF post-operatively and by current product labeling should be switched to 10 warfarin. This is especially true for a mechanical prosthetic valve. This labelling is based on the RE-ALIGN 11 sub-study of RELY-AF which demonstrated increased bleeding events in patients randomized to 12 dabigatran (79). Many cardiac surgeons would extend the prohibition of a DOAC for all cardiac surgeries. 13 Therefore if the patient is at high thrombotic risk post-procedure and anticoagulation is required then 14 warfarin is used. By convention this may be for 2 weeks or more which is within the period covered by 15 this document. A switch back to a DOAC could be reconsidered later but this timeframe is out of the 16 scope of this document. 17 18 19 Bleeding complications In the event of postprocedural bleeding complications, resumption of DOAC therapy will most often be 20 delayed until adequate hemostasis has been achieved. Clinical judgment and coordinated decision- 21 making by the primary management team and the proceduralist will be required to determine the 22 “best” time to restart DOAC therapy. When the DOAC is resumed, flexible dosing regimens (such as 23 starting with lower doses typically used for orthopedic VTE prophylaxis or with reduced renal function) 24 may be considered to reduce the possibility of further bleeding complications. Careful attention should 25 be given to ensuring that the patient resumes the most appropriate dose for stroke prevention in NVAF 26 as soon as possible. 37 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE Figure 5. Algorithm: How to Restart Anticoagulation 38 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 1 6. DISCUSSION AND IMPLICATION OF PATHWAY 2 3 The primary objective of this document was to provide a framework for the several decisions that need 4 to be made when managing a patient on anticoagulation that is undergoing a procedure. Management 5 of anticoagulation crosses over many different specialties. We have attempted to cite the literature to 6 offer direct guidance when possible and to highlight areas where clinical judgement is needed. As more 7 information becomes available, especially regarding the DOACs, many of these areas will be clarified in 8 the future. This is a clinical area of high volume, multiple hand-offs and potential risk. Hopefully this 9 document will aid in the management of our patients. 39 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE APPENDIX A: AUTHOR RELATIONSHIPS WITH INDUSTRY AND OTHER ENTITIES (COMPREHENSIVE)—ACC EXPERT CONSENSUS DECISION PATHWAY ON PROCEDURAL MANAGEMENT OF ANTICOAGULATION Committee Member Employment Consultant Speakers Bureau Ownership/ Partnership/ Principal Personal Research John U. Doherty, Chair None None None None None None Ty J. Gluckman William J. Hucker Jefferson Medical College of Thomas Jefferson University— Professor of Medicine Providence Heart and Vascular Institute— Medical Director Massachusetts General Hospital—Fellow in Cardiovascular Medicine James L. Januzzi Jr. Massachusetts General Hospital —Director, Dennis and Marilyn Barry Fellowship in Cardiology Research Cardiology Division; Harvard Medical School—Hutter Family Professor of Medicine Thomas L. Ortel Duke University Medical Center—Chief, Division of Hematology, Professor of Medicine and Pathology, Medical Director, Clinical Coagulation Laboratory Sherry J. Saxonhouse Carolinas HealthCare System— Cardiac Electrophysiology Associate Professor of Medicine, Sanger Heart & Vascular Institute Sarah A. Spinler Philadelphia College of Pharmacy, University of the Sciences—Professor of Clinical Pharmacy Expert Witness None Institutional, Organizational, or Other Financial Benefit None None None None None None None None Defendant, Class action litigation related to diabetes, 2015* None Critical Diagnostics* None Novartis* Phillips Roche* Sphingotec* None Provencio* Amgen (DSMB) Boehringer Ingelheim (DSMB)* Janssen Pharmaceuticals, (DSMB) None Instrumentation Laboratories None None None None None None None None None None None AstraZeneca None Boehringer Ingelheim Pharmaceuticals, Inc Daiichi-Sankyo None None None None None This table represents all relationships of committee members with industry and other entities that were reported by authors, including those not deemed to be relevant to this document, at the time this document was under development. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity, or ownership of ≥$5,000 of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted. Please refer to http://www.acc.org/guidelines/about-guidelines-and-clinicaldocuments/relationships-with-industry-policy for definitions of disclosure categories or additional information about the ACC/AHA Disclosure Policy for Writing Committees. *Significant relationship †No financial benefit 40 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE APPENDIX B: PEER REVIEWER RELATIONSHIPS WITH INDUSTRY AND OTHER ENTITIES —ACC EXPERT CONSENSUS DECISION PATHWAY ON PROCEDURAL MANAGEMENT OF ANTICOAGULATION [Peer Reviewer RWI table to be inserted after peer review is completed] 41 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE APPENDIX C: COMMON PROCEDURES AND ASSOCIATED RISK (This appendix, including contributions from external societies, is not included in our request for peer review) 42 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE APPENDIX D: ABBREVIATIONS AF = atrial fibrillation aPTT = activated partial thromboplastin time CrCl = creatinine clearance dTT = dilute thrombin time DOAC = direct-acting oral anticoagulant DVT = deep venous thombosis INR = international normalized ratio LMWH = low molecular weight heparin NVAF = nonvalvular atrial fibrillation OAC = oral anticoagulant PMAC = periprocedural management of anticoagulation TE = thromboembolic event TI = temporary interruption TIA = transient ischemic attack UFH = unfractionated heparin VKA = vitamin K antagonists VTE= venous thromboembolism 43 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE REFERENCES 1. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837-47. 2. Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949-53. 3. Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042-6. 4. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1-76. 5. Camm AJ, Lip GY, De CR, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719-47. 6. Friberg L, Skeppholm M, Terent A. Benefit of anticoagulation unlikely in patients with atrial fibrillation and a CHA2DS2-VASc score of 1. J Am Coll Cardiol. 2015;65:225-32. 7. Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. 8. Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500-10. 9. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263-72. 10. Olesen JB, Torp-Pedersen C, Hansen ML, et al. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost. 2012;107:1172-9. 44 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 11. Lip GY, Nielsen PB, Skjoth F, et al. The value of the European society of cardiology guidelines for refining stroke risk stratification in patients with atrial fibrillation categorized as low risk using the anticoagulation and risk factors in atrial fibrillation stroke score: a nationwide cohort study. Chest. 2014;146:1337-46. 12. Chao TF, Liu CJ, Wang KL, et al. Using the CHA2DS2-VASc score for refining stroke risk stratification in 'low-risk' Asian patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:165865. 13. Healey JS, Eikelboom J, Douketis J, et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation. 2012;126:343-8. 14. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e326S-e350S. 15. Sherwood MW, Douketis JD, Patel MR, et al. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation. 2014;129:1850-9. 16. Garcia D, Alexander JH, Wallentin L, et al. Management and clinical outcomes in patients treated with apixaban vs warfarin undergoing procedures. Blood. 2014;124:3692-8. 17. Beyer-Westendorf J, Gelbricht V, Forster K, et al. Peri-interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. Eur Heart J. 2014;35:1888-96. 18. Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131:488-94. 19. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-51. 20. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-104. 45 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 21. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-92. 22. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-91. 23. Chao TF, Wang KL, Liu CJ, et al. Age Threshold for Increased Stroke Risk Among Patients With Atrial Fibrillation: A Nationwide Cohort Study From Taiwan. J Am Coll Cardiol. 2015;66:1339-47. 24. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692-4. 25. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-47. 26. Kovacs RJ, Flaker GC, Saxonhouse SJ, et al. Practical management of anticoagulation in patients with atrial fibrillation. J Am Coll Cardiol. 2015;65:1340-60. 27. Eisen GM, Baron TH, Dominitz JA, et al. Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures. Gastrointest Endosc. 2002;55:775-9. 28. Perry DJ, Noakes TJ, Helliwell PS. Guidelines for the management of patients on oral anticoagulants requiring dental surgery. Br Dent J. 2007;203:389-93. 29. Veitch AM, Baglin TP, Gershlick AH, et al. Guidelines for the management of anticoagulant and antiplatelet therapy in patients undergoing endoscopic procedures. Gut. 2008;57:1322-9. 30. Malloy PC, Grassi CJ, Kundu S, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2009;20:S240-S249. 31. Anderson MA, Ben-Menachem T, Gan SI, et al. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc. 2009;70:1060-70. 32. Gogarten W, Vandermeulen E, Van AH, et al. Regional anaesthesia and antithrombotic agents: recommendations of the European Society of Anaesthesiology. Eur J Anaesthesiol. 2010;27:9991015. 46 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 33. Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med. 2010;35:64-101. 34. Zaca V, Marcucci R, Parodi G, et al. Management of antithrombotic therapy in patients undergoing electrophysiological device surgery. Europace. 2015;17:840-54. 35. Narouze S, Benzon HT, Provenzano DA, et al. Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications: guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med. 2015;40:182-212. 36. Burnett AE, Mahan CE, Vazquez SR, et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-32. 37. Witt DM, Clark NP, Kaatz S, et al. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:187-205. 38. Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120:2954-62. 39. Baron TH, Kamath PS, McBane RD. Management of antithrombotic therapy in patients undergoing invasive procedures. N Engl J Med. 2013;368:2113-24. 40. Liew A, Douketis J. Perioperative management of patients who are receiving a novel oral anticoagulant. Intern Emerg Med. 2013;8:477-84. 41. Heidbuchel H, Verhamme P, Alings M, et al. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2013;15:625-51. 42. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-33. 43. Birnie DH, Healey JS, Wells GA, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. 2013;368:2084-93. 47 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 44. Di BL, Burkhardt JD, Santangeli P, et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation. 2014;129:2638-44. 45. Cappato R, Marchlinski FE, Hohnloser SH, et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J. 2015;36:1805-11. 46. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with nonvalvular atrial fibrillation. Europace. 2015;17:1467-507. 47. Nin T, Sairaku A, Yoshida Y, et al. A randomized controlled trial of dabigatran versus warfarin for periablation anticoagulation in patients undergoing ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2013;36:172-9. 48. Grove EL, Hossain R, Storey RF. Platelet function testing and prediction of procedural bleeding risk. Thromb Haemost. 2013;109:817-24. 49. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395-401. 50. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151:713-9. 51. Gallego P, Roldan V, Marin F, et al. SAMe-TT2R2 score, time in therapeutic range, and outcomes in anticoagulated patients with atrial fibrillation. Am J Med. 2014;127:1083-8. 52. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-100. 53. Omran H, Bauersachs R, Rubenacker S, et al. The HAS-BLED score predicts bleedings during bridging of chronic oral anticoagulation. Results from the national multicentre BNK Online bRiDging REgistRy (BORDER). Thromb Haemost. 2012;108:65-73. 48 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 54. Pollack CV, Jr., Reilly PA, Eikelboom J, et al. Idarucizumab for Dabigatran Reversal. N Engl J Med. 2015;373:511-20. 55. Ansell JE. Universal, class-specific and drug-specific reversal agents for the new oral anticoagulants. J Thromb Thrombolysis. 2016;41:248-52. 56. Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med. 2015;373:2413-24. 57. Nutescu EA. Oral anticoagulant therapies: balancing the risks. Am J Health Syst Pharm. 2013;70:S3-11. 58. Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:286470. 59. Gage BF, van WC, Pearce L, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287-92. 60. Douketis JD, Healey JS, Brueckmann M, et al. Perioperative bridging anticoagulation during dabigatran or warfarin interruption among patients who had an elective surgery or procedure. Substudy of the RE-LY trial. Thromb Haemost. 2015;113:625-32. 61. Dunn AS, Spyropoulos AC, Turpie AG. Bridging therapy in patients on long-term oral anticoagulants who require surgery: the Prospective Peri-operative Enoxaparin Cohort Trial (PROSPECT). J Thromb Haemost. 2007;5:2211-8. 62. Jaffer AK, Ahmed M, Brotman DJ, et al. Low-molecular-weight-heparins as periprocedural anticoagulation for patients on long-term warfarin therapy: a standardized bridging therapy protocol. J Thromb Thrombolysis. 2005;20:11-6. 63. Malato A, Saccullo G, Lo CL, et al. Patients requiring interruption of long-term oral anticoagulant therapy: the use of fixed sub-therapeutic doses of low-molecular-weight heparin. J Thromb Haemost. 2010;8:107-13. 64. Spyropoulos AC, Turpie AG, Dunn AS, et al. Clinical outcomes with unfractionated heparin or low-molecular-weight heparin as bridging therapy in patients on long-term oral anticoagulants: the REGIMEN registry. J Thromb Haemost. 2006;4:1246-52. 49 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 65. Wysokinski WE, McBane RD, Daniels PR, et al. Periprocedural anticoagulation management of patients with nonvalvular atrial fibrillation. Mayo Clin Proc. 2008;83:639-45. 66. Austin JH, Stearns CR, Winkler AM, et al. Use of the chromogenic factor X assay in patients transitioning from argatroban to warfarin therapy. Pharmacotherapy. 2012;32:493-501. 67. Gullov AL, Koefoed BG, Petersen P. Bleeding during warfarin and aspirin therapy in patients with atrial fibrillation: the AFASAK 2 study. Atrial Fibrillation Aspirin and Anticoagulation. Arch Intern Med. 1999;159:1322-8. 68. Schulman S, Carrier M, Lee AY, et al. Perioperative Management of Dabigatran: A Prospective Cohort Study. Circulation. 2015;132:167-73. 69. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e278S-e325S. 70. Douketis J, Weitz JI, Murphy S eal. Perioperative adverse outcomes in patients with atrial fibrillation taking edoxaban or warfarin: analysis of the ENGAGE AF-TIMI 48 Trial. J Am Coll Cardiol. 2015;65:A2092. 71. Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood. 2012;119:3016-23. 72. Bristol-Myers Squibb Company and Pfizer Inc. Apixaban Prescribing Information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202155s000lbl.pdf. Accessed March 14, 2016. 73. Bayer Healthcare and Janssen Pharmaceuticals, Inc. Rivaroxaban Prescribing Information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022406s004lbl.pdf. Accessed March 14, 2016. 74. Daiichi Sankyo Inc. Edoxaban Prescribing Information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206316lbl.pdf. Accessed March 14, 2016. 75. Boehringer Ingelheim Pharmaceuticals, Inc. Dabigatran Prescribing Information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022512s017lbl.pdf. Accessed March 14, 2016. 50 EXPERT CONSENSUS DECISION PATHWAY: CONFIDENTIAL AND EMBARGOED – DO NOT CITE OR CIRCULATE 76. Feng W, Wu K, Liu Z, et al. Oral direct factor Xa inhibitor versus enoxaparin for thromboprophylaxis after hip or knee arthroplasty: Systemic review, traditional meta-analysis, dose-response meta-analysis and network meta-analysis. Thromb Res. 2015;136:1133-44. 77. Huisman MV, Quinlan DJ, Dahl OE, et al. Enoxaparin versus dabigatran or rivaroxaban for thromboprophylaxis after hip or knee arthroplasty: Results of separate pooled analyses of phase III multicenter randomized trials. Circ Cardiovasc Qual Outcomes. 2010;3:652-60. 78. Cohen AT, Spiro TE, Buller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513-23. 79. Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206-14. 51