Difference in Cp and Cv
advertisement
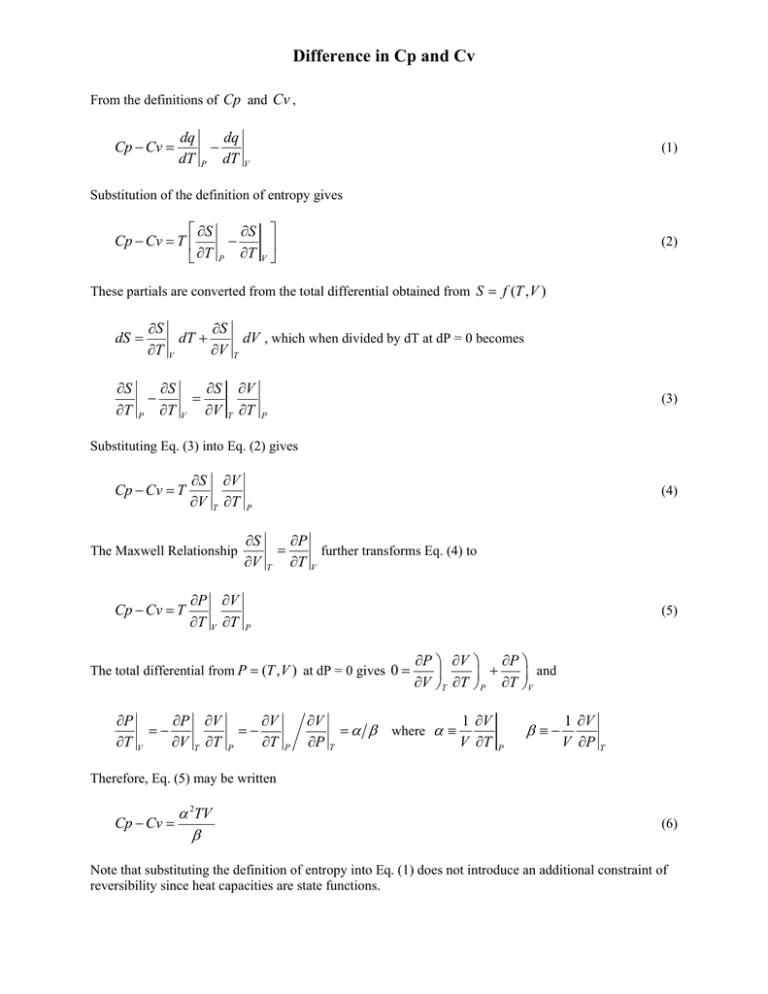
Difference in Cp and Cv From the definitions of Cp and Cv , Cp − Cv = dq dT − P dq dT (1) V Substitution of the definition of entropy gives ⎡ ∂S Cp − Cv = T ⎢ ⎣ ∂T ∂S ⎤ ⎥ ∂T V ⎦ − P (2) These partials are converted from the total differential obtained from S = f (T , V ) dS = ∂S ∂T ∂S ∂T − P dT + V ∂S ∂T = V ∂S ∂V ∂S ∂V dV , which when divided by dT at dP = 0 becomes T T ∂V ∂T (3) P Substituting Eq. (3) into Eq. (2) gives Cp − Cv = T ∂S ∂V T ∂V ∂T ∂S ∂V The Maxwell Relationship Cp − Cv = T (4) P ∂P ∂V ∂T V ∂T = T ∂P further transforms Eq. (4) to ∂T V (5) P The total differential from P = (T , V ) at dP = 0 gives 0 = ∂P ∂P =− ∂T V ∂V T ∂V ∂T =− P ∂V ∂T P ∂V ∂P ∂P ⎞ ∂V ⎞ ∂P ⎞ ⎟ ⎟ + ⎟ and ∂V ⎠T ∂T ⎠ P ∂T ⎠V = α β where α ≡ T 1 ∂V V ∂T β ≡− P 1 ∂V V ∂P T Therefore, Eq. (5) may be written α 2TV Cp − Cv = β (6) Note that substituting the definition of entropy into Eq. (1) does not introduce an additional constraint of reversibility since heat capacities are state functions.






