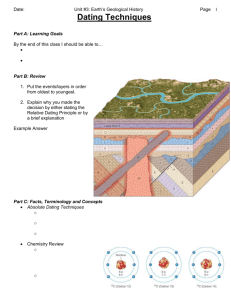
20. Carbon14Dating
advertisement

Carbon-14 dating: Teacher notes This is about how the carbon radioisotope C-14 is used to determine the approximate age of carbon-based items. These carbon-based items were once living, for instance, bones, hide, shells and wood. Carbon dating can also be used for wooden tools, wooden parts of buildings, and devices that are made from wood or other carbon-based material. The specific isotope which is being researched is Carbon-14, which has an atomic number of 6 but a mass number of 14 because it has two more neutrons than the most common, stable isotope of Carbon-12. Diagram of C-14 The element Carbon has isotopes with mass numbers 10, 11, 12, 13,14 and 15. These are known as C-10,C-11,C-12,C-13,C-14 and C-15. They have the following numbers of protons, neutrons and electrons: Isotope C-10 C-11 C-12 C-13 C-14 C-15 Protons 6 6 6 6 6 6 Neutrons 4 5 6 7 8 9 Electrons 6 6 6 6 6 6 The most abundant isotope is C-12 which is stable. C-13 is also stable, so neither C-12 nor C-13 experience decay of their nuclei. Here is the decay process for the radioactive isotope C-14 that is used to determine the age of once-living items. 1 of 3 Sci_Y09_U3_TN_Carbon14Dating Within 5730 years, half of the atoms of the C-14 isotope will experience decay of their nucleus, because the half-life of C-14 is 5730 years. Carbon-14 decays by a beta decay process where one of the neutrons becomes a proton. Because the atom now has one more proton it becomes nitrogen N -14, which is stable. So the products of decay are N-14 and beta particles, or electrons. As the neutron changes into a proton, it changes from a neutral charge to positive, so a beta particle with a negative charge is emitted. This is an electron. Although the mass number is still 14, each atom that has decay of the nucleus now has 7 protons. This means it has a different atomic number, making it N-14, a stable isotope of a different element, Nitrogen. Because of the beta particle it now has 7 electrons, the same as the number of protons. The remains of living organisms contain much carbon, including a small proportion of Carbon-14. The scientist who invented carbon dating was Willard F. Libby who was working with fellow researchers at the University of Chicago. Summary of the process of carbon dating •• Organisms consume both C-12 and a very small percentage of the C-14 isotope which occurs naturally by cosmic rays converting N-14 in the atmosphere to C-14 in carbon dioxide. •• While organisms are alive, C-14 that decays is replenished with new C-14 in nutrients ingested. •• Once they die, the amount of a C-12 in the remains of the organisms stays the same but the C-14 decays slowly to N-14. •• Every 5730 years half of the remaining C-14 decays. •• To date an item, scientists measure the proportion of C-14 in a sample compared with C-12. •• They compare this proportion with the proportion of C-14 in a sample of the same item that is from the present day. •• They use this proportion to calculate the age of the dating sample. 2 of 3 Sci_Y09_U3_TN_Carbon14Dating For example: •• If the dating sample has half the proportion of C-14 compared to the present-day sample, it is around 5730 years old. •• If it had a quarter of proportion of C-14 compared to the present-day sample, this would indicate that the dated sample is approximately two half-lives of Carbon-14 old, so it would be around 11460 years old. •• If the proportion C-14 was only 1/16 of the proportion found in the present-day sample, then the dating sample would be around three half-lives of C-14 old, which is 17190 years. The reasons that C-14 is the best carbon isotope to use for this dating are: •• Carbon appears naturally in all living things, so will be available in their remains. •• The stable isotopes of carbon do not decay, so the proportion of C-14 remaining will change over time when compared to C-12. •• Both other naturally-occurring isotopes of carbon are stable and do not decay. •• Of the radioactive isotopes of carbon, C-14 is the isotope that has a half-life long enough to use for dating organic items. All three of the others have half-lives less than 30 minutes. •• Because the other radioactive isotopes of carbon do not occur naturally, they would not be expected to be found in the remains of organisms. Carbon dating is one example of dating techniques using a radioactive isotope. These techniques might be used by archaeologists, geologists, geophysicists and other scientists dealing with the earth. Bibliography: Website article: C-14 dating, Iowa State University, Ames, Iowa. http://www.ndt-ed.org/ EducationResources/CommunityCollege/Radiography/Physics/carbondating.htm 3 of 3 Sci_Y09_U3_TN_Carbon14Dating


![[month, day, year] Fire Department of New York 9 MetroTech Center](http://s2.studylib.net/store/data/013281229_1-e481fc7617a1140ba1d71e7252f57e68-300x300.png)



