C. J. Ho Department of Mechanical Engineering National Cheng
advertisement

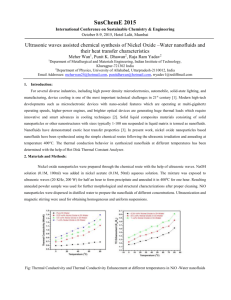
C. J. Ho Department of Mechanical Engineering National Cheng Kung University Tainan, Taiwan 70101, ROC 1 Functional(Intelligent) Media Functional medium may be classified into: a. One that has an ability to adapt its functionality in terms of its properties (chemical, electrical, magnetic, mechanical, or thermal) in response to a change in external stimuli in the surrounding environment. b. One that transforms energy from one form to another by means of its behaviors: • • • • Photovoltaic Thermoelectric Piezoelectric Photo‐luminescent 2 Functional(Intelligent) Media Multidisciplinary research interests for engineering applications including: ¾ Structural health monitoring ¾ Smart manufacturing ¾ Active fluid, thermal, vibration, deformation control ¾ Intelligent sensory actuator ¾ Energy conversion and management ¾ Biomedical material 3 Functional (Complex/Multiphase) Fluids • A mixture of different substances or different phases of matter (solid, liquid, or gas) in coexistence. • Functional fluids may appear as: ¾ Dispersed regimes (i.e. not materially connected), e.g. particle, droplet or bubbly flows ¾ Non‐dispersed regimes, e.g. flows through a porous medium 4 Examples of Functional Fluids y Electro‐Rheological (ER) fluids y Magneto‐Rheological (MR) fluids y Photo‐Rheological (PR) fluids y Magnetic fluids y Viscoelastic fluids y Viscoplastic fluids y Thermal fluids y Others 5 Functional Thermal Fluids High‐density thermal energy Wide temperature range Heat transfer enhancement High heat capacity Functional Thermal Fluids Down‐sizing of heat exchanger Flow drag reduction Reduction in heat loss High‐speed transport of high‐density thermal energy Classification of Functional Thermal Fluids Functional Thermal Fluids Sensible‐heat Fluids Latent‐heat Fluids e.g. e.g. Coolants, Refrigerants Solid‐liquid phase change materials (PCM) Absorbent of absorption refrigeration system Aqueous polymer solution Vapor‐liquid phase change materials Aqueous surfactant solution Nanofluids 7 Nanofluids ‐ suspensions of nanoparticles in base fluids Conventional suspensions that contain mm‐ or μm‐ sized particles do not work with the emerging “miniaturized” technologies because they can clog the tiny channels of these devices. Modern nanotechnology provides opportunities to produce nanoparticles of various materials. Argonne National Lab (S. U. S. Choi’s team, 1995) developed the novel concept of nanofluids. 8 Materials for Nanoparticles and Base Fluids Nanoparticles : Oxide ceramics – Al2O3, CuO, TiO2 Base fluids : Water Ethylene‐ or tri‐ethylene‐ glycols and other coolants Metal carbides – SiC Nitrides – AlN, SiN Oil and other lubricants Metals – Ag, Au, Cu, Fe Bio‐fluids Nonmetals – Graphite, carbon nanotubes Polymer solutions Other common fluids Layered – Al + Al2O3, Cu + C PCM – S/S Functionalized nanoparticles 9 Nanofluids in Nature and Industry Nature is full of nanofluids, like blood, a complex biological nanofluid where different nanoparticles (at molecular level) accomplish different functions Many natural processes in biosphere and atmosphere include wide spectrum of mixtures of nanoparticles with different fluids Many mining and manufacturing processes leave waste products which consist of mixtures of nanoparticles with fluids A wide range of self‐assembly mechanisms for nano‐ structures start from a suspension of nanoparticles in fluid 10 Applications of Nanofluids Heat‐transfer nanofluids Tribological nanofluids Surfactant and coating nanofluids Chemical nanofluids Process/extraction nanofluids Environmental (pollution cleaning) nanofluids Bio‐ and pharmaceutical‐nanofluids Medical nanofluids (drug delivery and functional tissue‐cell interaction) 11 Attracting Features of Heat Transfer Nanofluids z Abnormally increased thermal conductivity at low nanoparticle concentrations z Strong temperature‐dependent thermal conductivity z Non‐linear increase in thermal conductivity with nanoparticle concentration 12 Enhanced Nanofluid Thermal Conductivity Comparison of experimental data on thermal conductivity of nanofluids, Int. J. Thermal Sciences, vol. 46, pp. 1-19, 2007. 13 Thermal Conductivity Ratio knf/kbase Nonlinear Increase in Conductivity with Nanotube Loadings 1.08 1.06 1.04 1.02 1.00 0.0 0.4 0.8 1.2 Volume Fraction [%] Measured and predicted thermal conductivity enhancement for nanotube-in-oil nanofluids. Appl. Phys. Lett. 79, 2252, 2001. 14 Temperature‐Dependent Conductivity 1 .3 A l 2 O 3 (1 % ) A l 2 O 3 (4 % ) Thermal conductivity ratio λ/λ wate 1 .2 5 1 .2 1 .1 5 1 .1 1 .0 5 1 0 10 20 30 40 50 60 T e m p e ra tu re ( C) Temperature dependence of thermal conductivity enhancement for Al2O3-in-water nanofluids J. Heat Transfer, 125, 567, 2003. 15 Disparity between Model Predictions and Experimental Data on Thermal Conductivity for Nanofluids Comparison between selected theoretical predictions and experimental data on thermal conductivity for Al2O3-water nanofluids. Int. J. Thermal Sciences, vol. 46, pp. 1-19, 2007. 16 Possible Mechanisms for Enhanced Thermal Conductivity of Nanofluids Enhancement of thermal conductivity due to (a) nano-layer of liquid structure at liquid-particle interface; (b) ballistic & diffusive phonon transport in a solid particle; (c) highly conducting clusters. Int. J. Heat Mass Transfer, vol. 45, pp. 855-863, 2002. 17 Normalized conductivity (keff/kBF) Brownian Motion of Nanoparticles Water+ Cu (6nm) 1.8 1.6 1.4 Water+ Al2O3 1.2 (38.4nm) 1.0 300 305 310 315 320 325 Temperature (K) Temperature-dependent thermal conductivities of nanofluids at a fixed concentration of 1 vol.%, normalized to the thermal conductivity of the base fluid. Appl. Phys. Lett. , 84, 4316, 2004. 18 Applications of Heat Transfer Nanofluids Efficient flow and lubrication, cooling and heating in new and critical applications, like electronics, nuclear and biomedical instrumentation and equipments, transportation and industrial cooling, e.g. cooling densely packed integrated circuits at the small scale to heat transfer in nuclear reactors at the large scale. Thermal management in various critical applications, as well as environmental control and cleanup, bio-medical applications, and directed self-assembly of nanostructures, which usually starts from a suspension of nanoparticles in fluid. 19 Heat Transfer Performance of Al2O3‐Water Nanofluid in a Micro‐Channel Heat Sink Dc (μm) Dbase (mm) Hch (μm) Lch (mm) Wch (μm) Wfin (μm) 10 24 800 50 283 300 20 Experimental Loop for Heat Transfer Performance of a Micro‐Channel Heat Sink 21 Heat Transfer Efficacy of Nanofluid for Simultaneous Developing Flow in a Channel The average Nusselt number for the simultaneous developing flow in an isothermal channel can be evaluated by a correlation due to Seider and Tate as Nu D = 1.86( Re D Pr 1/ 3 μ 0.14 ) ( ) μs Lch / Dh Assuming (μ/μs) ≈ 1, a relation of the average heat transfer coefficient with the relevant thermophysical properties of the fluid as well as the characteristic lengths of the channel can then be expressed as h ~ m 1/ 3c1/p 3 k 2 / 3 Dh−1 L−ch1/ 3 22 Thermophysical Properties of Nanoparticle, Base fluid, and Nanofluid at 30°C Properties Nanofluid (alumina-water) Nanoparticle Base fluid (alumina) (water) φ = 1 vol.% 2 vol.% 3600 995.1 1021.1 1047.2 4.144 (Eq. (3a)) 4.110 (Eq. (3a)) 4.058 (Eq. (3b)) 3.943 (Eq. (3b)) 0.635 0.654 ρ (kg/m3) c p (kJ/kg⋅K) 0.765 k (W/m⋅K) 4.178 36.0 0.620 μnf ×103 (N⋅s/m2) φ T = 20°C 25°C 30°C 35°C 40°C 0 vol.% 0.9590 0.8550 0.7690 0.6950 0.6310 1 vol.% 1.0040 0.8955 0.8062 0.7483 0.6507 2 vol.% 1.0930 1.0750 0.9689 0.8750 0.7947 23 Enhanced Nusselt Number of Nanofluid 1.8 25 20 ϕ (Vol.%) 0 Symbols A 1.7 B 1.6 1 2 1.5 15 1.4 hnf / hbf1. Nu | 1.3 10 1.2 1.1 1 vol. % 2 vol. % 1 5 200 500 Re 1000 1500 2000 0.9 200 400 600 800 1000 1200 1400 1600 Re( ρbf / ρ nf )( μnf / μbf ) 24 1800 Hydraulic & Thermal Performances of Al2O3‐Water Nanofluid in a Micro‐Channel Heat Sink 0.1 1.0 φ (%) Present work Lee & Mudawar [14] 0.08 Ritd (K/W) 0 1 2 ϕ (Vol.%) 0 1 2 0.06 Symbols A B 0.04 0.02 0.1 f 0 Rlm (K/W) 16/Re 0.01 60 Re 500 1000 1500 2000 0.04 0.02 0 0 0.2 0.4 0.6 0.8 P(W) Ritd = (Tw − Tin ) qf Rlm = ΔTlm qf 25 Solid-Liquid Phase Change Material (PCM) Suspension 26 Latent Heat Functional Thermal Fluids & Their Applications Latent Heat Functional Thermal Fluids Micro-emulsion/microencapsulated PCM (paraffin wax) suspensions Ice slurry Clathrate slurry (host gas or liquid, refrigerant) Applications Thermal management Air conditioning Cooling Refrigeration Air conditioning 27 PCM (Phase Change Material) Suspension y Suspensions of micro‐ or nano‐sized particles of solid‐liquid phase change material (PCM), by means of emulsion and/or micro‐encapsulation techniques, in a suspending fluid such as water, ethylene glycol. y Concept originated in the early 1980 for convective heat transfer enhancement. y Functional thermal fluid of dual capability of sensible and latent heat transport. 28 Thermally Developing Forced Convection in a Circular Duct PCM Particles Liquid Solid Suspending Fluid r+ PCM Suspension 0 Flow lu+ Adiabatic ri+ x+ lh+ ld+ qh′′ Adiabatic 29 Experimental Set-Up 30 Heat Transfer Efficacy of PCM Suspension for Thermal Developing Flow in a Channel The average Nusselt number for the thermal developing flow in a channel can be evaluated by a correlation of the form: Nu = C ( Re Pr 1/ 3 ) + + lh / 2ri A relation of the average heat transfer coefficient with the relevant thermophysical properties of the fluid as well as the characteristic lengths of the channel can then be expressed as h ~ Q 1/ 3 ρ 1/ 3c1/p 3 k 2 / 3 (ri + ) −1 (lh+ ) −1/ 3 31 Thermophysical Properties of PCM particles, Base fluid, and Suspensions at 30°C Description/Compo sition Density (kg/m3) Specific heat (J/kg⋅K) Thermal conductivity (W/m⋅K) Dynamic viscosity (kg/m⋅s) Pure water 997 4180 0.62 8×10-4 n-Eicosane 856(solid) 778(liquid) 2210 0.15 (solid) 0.35 (liquid) - Urea-formaldehyde 1500 1672 0.42 5×10-5 MEPCM particle 961.4 2135.7 0.2 - 2% suspension 991 4129 0.608 9.3×10-3 5% suspension 982 4061 0.592 1.05×10-3 10% suspension 970 3941 0.567 1.26×10-3 32 Model Prediction vs. Experimental Data for Outer Wall Temperature o * Re = 200 , Ste = 0.1 , θin = - 0.07 , cv = 10 % 1 46 Experiment [ 5 ] ( tw = 0.52 ) Present prediction ( tw = 0.52 , k*wf = 648.2 ) Present prediction ( tw = 0 ) Prediction [ 5 ] ( tw = 0 ) Prediction [ 6 ] ( tw = 0 ) 0.8 qh = 20W , Tin ≈ 33.5 C , m ≈ 60 g/min 44 cm ( % ) Water 1% 5% experiment prediction 42 θi-θin Tw,o ( oC ) 0.6 0.4 40 38 36 0.2 34 0 0 0.02 0.04 + 0.06 + i x / ( r × Pef ) 0.08 0.1 0.12 32 -10 0 10 20 X (cm) 30 40 33 Bulk Temperature θb & Mean Particle Melted Fraction ξb 34 Duct Wall Temperature θw 2 1.5 Pef = 1000 cv = 2% cv = 5% cv = 10% 0.5 * Ste = 0.5 Ste* = 0.5 0.4 cv = 0 % Ste* = 0.1 Ste* = 0.1 1 Ste* = 0.01 θw θw 0.6 cv = 2% cv = 5% cv = 10% Pef = 100 cv = 0 % 0.3 0.2 0.5 0.1 0 * Ste = 0.01 0 0 0.1 + 0.2 + i x / ( r × Pef ) 0.3 0.4 0 0.01 + 0.02 + i 0.03 0.04 x / ( r × Pef ) 35 Local Nusselt Number Nub 50 60 Pef = 100 cv = 2% cv = 5% cv = 10% 40 Pef = 1000 cv = 2% cv = 5% cv = 10% 50 * 40 Ste* = 0.1 * 30 Nub Ste = 0.5 * Nub Ste = 0.5 Ste = 0.1 30 * Ste = 0.01 20 Ste* = 0.01 10 0 20 10 cv = 0 % ( 4.36 ) x+ / ( r+i× Pef ) cv = 0 % ( 4.36 ) 0 0.1 0.2 + + i 0.01 0.02 x / ( r × Pef ) 36 Sensible & Latent Heat Transport Fractions 1 1 cv = 2% cv = 5% cv = 10% * Ste = 0.1 1 0.8 0.8 0.6 0.6 0.6 0.6 0.4 0.4 0.4 0.4 0.2 0.2 0.2 0.2 101 102 Pef 103 * * 0 100 * 0.8 ( qh,conv )sen 0.8 0 104 0 100 101 102 Pef 103 0 104 * qh*,conv N Total convection heat transfer rate 1 1 ρ pf cv [(θb ( x = lh ) − θ b ( x = 0)] + [ξ b (x = lh ) - ξ b (x = 0)] = * * 4lh 4lh ρbf Ste Sensible heat transport fraction (q*h,conv ) sen ( qh,conv )lat * Ste = 0.01 ( qh,conv )lat ( q*h,conv )sen 1 cv = 2% cv = 5% cv = 10% Latent heat transport fraction (q*h,conv )lat 37 Heat Transfer Enhancement Effectiveness 4 3.5 3 cv = 0 % cv = 2 % cv = 5 % cv = 10 % 3 Ste* = 0.01 2.5 * Ste = 0.1 cv = 0 % cv = 2 % cv = 5 % cv = 10 % 2 εh εh 2.5 2 1.5 1.5 1 1 0.5 100 101 εh = ( 102 Pef 103 h Nu b * )(k bf ) = b Nu f hf 104 0.5 100 101 102 Pef 103 104 (Heat transfer enhancement effectiveness) 38 Heat Transfer/Pumping Power Performance Index 3 3 Pef = 10 Pef = 100 Pef = 1000 2.5 2.5 2 Ste* = 0.01 1.5 * Ste = 0.01 εh / εp εh / εp 2 cv = 2 % cv = 5 % cv = 10 % * Ste = 0.1 1 1.5 1 Ste* = 0.1 0.5 0 0.5 0 0.02 0.04 0.06 cv 0.08 0.1 h Nu b * εh = ( )(k bf ) = b Nu f hf 0.12 0 100 εp = 101 102 Pef 103 104 [ Δp( m /ρ )]b (Pumping power ratio) [ Δp( m /ρ )] f 39 Uncertainties and Challenges for Applications of Functional Thermal Fluids: Disparities exist between experimental results from different studies concerning heat transfer efficacy of using nanofluids for various heat transfer configurations or the data of thermophysical properties for the functional thermal fluids. Stability of nanoparticles/PCM suspension, including clustering, agglomeration, clogging, and so on. Necessity of developing standardized method for experimental formulation of various functional thermal fluids. Development of theoretical models and experimental methods for characterizing nanoscale structure and dynamics functional thermal fluids in the laboratory and in nature. Further understanding of heat and mass transport phenomena and thus development of theoretical models for functional thermal fluid flows including physical and chemical interactions between nanoparticles/PCM and base‐fluids. Identifying new and unique applications for functional thermal fluids. 40 Thanks for Your Attentions 41