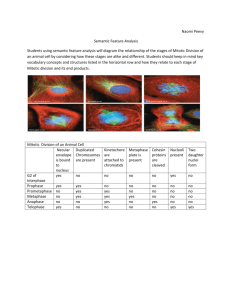
I@IIll @ III@III@IIII @II@@I@IIII@I @OHI@III
advertisement

CANCER VOLUME 25 RESEARCH NOVEMBER 1965 NUMBER 10 Mitotic and Functional Homeostasis: A SpeculativeReview w. S.BULLOUGH Birkbeck College, University of London, London, England CONTENTS I. Introduction II. Theories of gene expression A. Genetic control mechanisms B. Embryonic differentiation C. Cell division and cell function III. Mitotic honteostasis in adult tissues A. Control by chalones 1684 1684 1684 1685 1686 1687 1687 B. The role of the adrenals 1687 1. The diurnal mitotic cycle 2. Adrenalin and the glucocorticoid 3. Conclusions C. The wound reaction 1687 1688 1689 1689 hormones D. Regeneration 1690 1. Local and general mitotic reactions 2. Hormones and regeneration 3. Chalones and regeneration 4. The experimental evidence 5. Mitotic inhibition and stimulus 6. The effects of metabolic damage 7. The effects of the adrenal hormones 8. Other evidence on regeneration 9. Conclusions E. The mitotic cycle 1690 1691 1692 1692 1693 1693 1694 1695 1695 1695 1 . The phases of the cycle 1695 2. The initiation of the prosphase 3. The phase of DNA synthesis 4. Antephase and mitosis 5. RNA and protein synthesis 6. The action of the chalone complex F. Conclusions 1696 1696 1696 1697 1697 1698 Iv. Mitotic and functional homeostasis A. The basic mechanism 1. 2. 3. 4. 1699 1699 Mitosis and cell function Life expectancy of cells The nature of the dichophase Conclusions B. The versatility 1. Methods 1699 1700 1701 1702 of the mechanism of alteration 1703 of tissue mass 1683 @ 1703 This One I@ @ III@III@IIII IIll@II@ @I I@IIII@ @OH 2C17-TSN-2XGG Vol. 25, November Cancer Research 1684 1703 1704 1707 1708 1709 1710 1710 1711 1712 1712 1713 1714 1715 1715 1716 1716 1716 2. The mitogenic hormones 3. The poietins 4. Mitotic control in lymphoid tissue 5. The growth of hair 6. Conclusions C. The breakdown of the mechanism 1. The mechanism itself 2. Carcinogenesis 3. The chalone complex and the latent period 4. Promoting agents 5. Retarding agents 6. Conclusions V. General summary and conclusions A. The role of inducing substances B. The role of the chalones C. The role of the hormones D. The problem of gene damage II. THEORIES I. INTRODUCTION One of the most important cerns both the manner tissues and the manner entiation is maintained In recent steps problems con have taken an understanding a number of this problem, of new and al though much of what is now being written is admittedly hypothetical, it nevertheless seems worthwhile at this moment to attempt to review these new ideas and to place them in a logical context. Perhaps the most significant of recent biologic discov eries in this field have been those that have provided new knowledge and understanding of the genetic manipula tion of protein synthesis (see, for instance, Ref. 262), of the changes that occur in the expression of the genome when an embryonic cell differentiates (see, for instance, Refs. 165, 166), and of the homeostatic mechanisms by which both specialized function and mitotic activity are controlled in the differentiated cells of adult mammals (see, for instance, Refs. 73, 75). These 3 fields of research are evidently closely interrelated, and the conclusions that have emerged probably apply generally to all metazoan animals. However, has been restricted sues of adult expression controlled in the present to the situation mammals review consideration that exists in the tis and to the manner in which the of the genome in a differentiated cell may be by mitotic and functional homeostatic mecha nisrns. It appears these self-evident. mechanisms the problem must of cancer, that have and indeed that understanding of the changes genesis is likely to be achieved previous knowledge sues maintain their any conclusions important in little or no real occurring during carcino without some considerable of the manner organization. regarding implications in which normal It seems reasonable Received for publication February 4, 1965. The most important biologic discoveries of recent years have concerned the structure of the DNA' molecule, and the most important hypotheses have concerned the possi ble manner in which genetic information may be coded in the base sequences of this molecule. This work is outside the scope of the present review except in so far as it forms the background for our understanding, first, of the man ner in which instructions may emanate from the nucleus t.o induce the synthesis of specific types of proteins, and second, of the manner in which, from time to time, these instructions may be changed to take account of the changed conditions, whether inside or outside the cell. Regarding these 2 points, most of our information has been derived from studies of microorganisms ; recent reviews are those of Monod el al. (262, 355, 356), whose conclusions have been discussed in a more general chemical context by Dean and Hinsheiwood (131). There are, however, strong indications that similar mechanisms must also exist in the differentiated cells of adult mammals, in which they may act as an essential part of homeostatic mechanisms. As regards microorganisms, current hypotheses may be summarized as follows. Two main types of genes are recognized : the structural genes and the regulator genes. A structural gene, acting through the intermediary of an RNA messenger, is stimulated to initiate the synthesis of a particular protein. The stimulus to commence activity originates in a particular region of the gene called the “operator,―and each operator may be in control of 1 or of several related structural genes. Those genes, whether 1 or many, that are under the control of a single operator are together known as an “operon.― When an operon tis to conclude that the main, and perhaps only, reason why cancer has remained such a mystery for so long is our lack of any adequate understanding of these normal mecha nisnis. OF GENE EXPRESSION A. GENETICCONTROL l\1ECHANISMS to form in which, in such tissues, differ and mitotic activity is controlled. years biologists towards of all biologic in which cells differentiate 1965 contains several structural genes, these are usually adjacent to each other so as to form a cluster on the chromosome (134). Each opei ator, and therefore each operon, is under the ‘The following abbreviations are used : DNA, deoxyribo nucleic acid; RNA, ribonucleic acid; ACTH, adrenocorticotropin; ATPase, adenosine triphosphatase; ATP, adenosine triphosphate. BULL0uGH—Mitotic and Functional negative control of a substance called a “repressor,― which may be a protein. Each repressor, which acts only on a specific operator, is produced in response to the activity of a regulator gene, probably through the normal iries senger RNA mechanism. It is because the repressor has the ability to react with particular substances, which in the case of microorganisms are metabolites of low mo lecular weight, that the whole system is so remarkably versatile in its response to the needs of the moment . A metabolite that reacts with a repressor in this way is called an “effector―; in the reaction the repres.sor is either inactivated, so that the relevant messenger RNA and proteins begin to be synthesized, or it is activated, so that the synthesis of messenger RNA and protein is inhibited. Thus in microorganisms the expression of the genoine is determined by the type of metabolite available, and it is important to note that the reaction to a new situation is almost instantaneous. A reaction time of less than 15 sec has been established (262). It is interesting to note that an effector of this type may not necessarily exert its influence on the repressor by binding at the same active site as that which binds the operator. A recent hypothesis suggests the possibility that a repressor may be an allosteric protein, which possesses 2 separate active sites (355). In this situation the activation or inactivation of the repressor is considered to depend on the molecular distortion caused when the effector becomes bound to its own site. Because of this an effector need not bear any obvious chemical relation ship to the synthetic pathway it influences. Although these conclusions and suggestions relate almost entirely to the reactions of unicellular organisms to sub strates, they are also obviously relevant. to any consider ation of cellular activity in metazoans. In spite of the fact that in the case of the metazoans the evidence is only slight, Monod and Jacob (356) have concluded that it may still be sufficient to “justifythe conclusion that the rate of structural gene expression is controlled, in higher organisms as well as in bacteria and bacterial viruses, by closely similar mechanisms, involving regulator genes, apore pressors, operators and operons.― Some of the still in adequate mammalian evidence relates to the control of the agouti hair pattern (43, 118) and to certain liver enzymes Homeostasis process of differentiation on the expression 1685 and also the effect it. must have and control of the genome. The nucleus of a fertilized egg contains a gene comple ment. that is caj)able of directing the construction of each and every part of the fully differentiated adult; in other words, the genome is totipotent. It. is also evident, at least iii some animals such as molluscs (408) and am l)hibialls (164, 165), that certain cytoplasmic constituents that. may originate in the cortex (126) of the newly ferti lized egg are already so organized and distributed as to predetermine the positions of such major adult organs as brain, intestine, or muscles, and that as the egg undergoes repeated cleavage this basic patterti becomes even more firmly stabilized by the developing cell walls (133, 167). It is also evident that as development proceeds the cyto l)lasmic cOmI)onents themselves undergo increasing spe cialization and diversification. Thus the cells in each region of the developing body are characterized by a particular tyj)e of cytoplasm, and it has beeii suggested that it is the contents of this which, by reacting with the genonie, determine the type of different i at.ion that will occur. It is interesting that in the higher plants differentiation situations is also evidently determined in which the cells find themselves by the (460). One of the methods by which cytoj)laslmc may be induced, so that. it in turn may specializat determine ion the pattern of differentiation within a group of cells, is through the influence exerted by son@e neighboring preformed tissue. Thus it is commonly said that one tissue will induce the appearance of another (see Refs. 231, 430, 431). Such a tissue interaction may be envisaged as involving either a stimulus or an inhibition : in the sense of a stimulus it may be considered that the differentiating cells, following intrinsic genotypic and amplify these instruct ions instructions ‘ ‘select among, translate in response to extrinsic influences― (213) ; in the sense of an inhibition it is possible to regard the reaction as the outcome of the suppression of intrinsic genotyj)ic instructions when the “products of differentiating tissues act. as inhibitors of like activities in surrounding tissues― (50). Some indication of the com plexity of the induction process is given by Saxén(430), who stresses that the inducing factors may act as triggers only and that the main part of the differentiation mecha (315, 367, 392). The existence of typical messenger R.NA in the mammal has also been demonstrated in association with the ribosomes in rat liver cytoplasm (300, 362). However, apart from the existence of this evidence, it This situation is immediately reminiscent of the effect or repressor-operator type of mechanism described in micro organisms. The simplest suggestion would be that. in seems embryonic most improbable from what is already known of the remarkable uniformity of the biochemical organization in all living matter that the mechanisms of gene expression and gene control will prove to be fundamentally different in the cells of the various groups of organisms, even when these are as different as mammals and bacteria. B. EMBRYONIC DIFFERENTIATION In considering metazoan animals it is obvious that, apart from their multicellular nature, their outstanding peculiarity lies in the manner in which their component cells become differentiated and in the way in which these differentiated cells are grouped together to form tissues. It is extremely important to determine the nature of the IlisIfl evidently lies inside the reacting cells all the specialized cells. genot.ypic instructions remain suppressed until, during differentiation, one spe cific repressor is inhibited by a particular effector. If this suggestion is valid, then, at least in the higher animals, it must also be accepted that this proces@s involves the permanent suppression of all or most of the other spe cialized potentialities of the genome (53, 166). It is interesting to note that in the higher plants it is possible to stimulate a single fully differentiated cell to such a degree that it will give rise to a new plant (460), and this imist indicate that within such a differentiated cell the genome is still potentially totipotent. However, there is at the moment no evidence at all that differentiated mammalian cells, with which this review is primarily con certied, can act in this way. In such cells differentiation commonly appears to be irreversible. From what has been said it is clear that differentiation is a irocess that, in mammals, takes place almost entirely in the embryo. Once organogenesis is complete all the cells of the body, except. for instance the germ cells and the stem cells of the hemopoietic systems (see p. 1706), are specialized to such an extent that each must continue ever afterwards to breed true to type. It is with this type of differentiated concerned, that cell that the present review and in such a cell it is the genetic remain after differentiation iml)ortance. It now appears that that maliaii tissues the only remaining functional genes are those that are of outstanding functional dictate Unfortunately, in the literature is mainly potentialities in most adult or potentially it is common in the manner of the are regarded to which they belong for to find that. only those cells that fuiiction tissue of the pathways, typical of their genome has become severely Except and CELL DIVISION AND CELL FUNCTION for a few highly specialized mitosis or in protein synthesis for function may take the forni of a diffusible chemical n@essenger (377), which may act. as part of a negative feedback mechanism to suppress mit.osis within the cells concerned (259, 348). In the language of the microbiologists such a substance might be considered to be an effector. as.sociat.ed with chromosomes Stedman and St.edman are capable of suppressing still remains whether they RNA unanswered synthesis. (see Refs. The 5, 24, 504), but it is already clear that the histones are not as tissue specific as was originally thought and as would be necessary if they were to form an integral part of a tissue-specific control mechanism (272). In the 2nd line of research, Bullough and Laurence (86, 87) have extracted from mouse epidermis a substance that inhibits epidermal mitosis and has the necessary characteristics to act as part of a negative feedback mecha nism, and they have also obtained evidence indicating that similar tissue-specific mitotic inhibitors are associated with the hypodermis and the hair bulbs (82). In addition, tissue-specific mitotic inhibitors have been extracted by Saetren cells such as neurones but also exist free in the cell. (458) questioned may be concerned in the tissue-specific control of gene expression, and Huang and Bonner (253) have shown that they typically limited. For an excellent discussion of the meaning of the term “differentiation―see Weiss (513). C. which the commencement of 1 type of specialized synthetic activity may be accompanied by the cessation of another. On theoretical grounds it has been thought for some time that. an integral part of the control mechanism that de termines whether a cell indulges in protein synthesis for question as differ entiated. Cells that belong to the tissue but have never functioned in the typical manner are described as undiffer entiated, while cells that have ceased to be able to function in the ty@)ica1 manner are described as dedifferentiated. In fact., almost all the somatic cells of an adult mammal, whatever may be their appearance or function, must be regarded as fully differentiated in the sense that the power exl)res.sion 1965 Recently 2 lines of research have converged on this problem. In the 1st, there has been an investigation of the possible functions of the histones, which not only are main the synthesis enzymes needed for the general metabolic mitotic activity, and for tissue function. of Vol. 25, November Cancer Research 1686 (425) from liver amid kidney and by Rytomaa and and for a number of aging cells such as circulating erythro cytes and granulocyt.es, the tissue cells in an adult mammal are commonly capable of at least 3 basic programs of protein synthesis, of which 1 is essential while the others Kiviniemi (423) from mature granulocytes. All these substances have similar physical properties, and Bullough (73) has proposed that they should be called chalones, a appear are discussed to be optional. The essential program ensures a name that refers to their inhibitory in detail below actions. ; here These it is necessary actions only continuous supply of the enzymes needed in such vital metabolic l)ath\vays as that of the tricarboxylic acid cycle (125, 169, 363). In this connection it is interesting to note that Davidson et a]. (129) have shown that, when stress that they result in the suppression of synthesis mitosis within the tissues in which they originate. whereby a choice actinomycin programs, it must D is added to connective to block the synthesis of messenger enzymes as succinic dehydrogenase tissue RNA, cells in vitro such continue generalized their action unaffected. This suggests that essential enzymes may not be subject to any immediate genetic control, which may indicate either that they have an unusually long functional life or that their synthesis is dictated at the ribosome level and needs no repeated genetic stimulus for its continuance. By contrast, synthesis synthesis are the other highly of those 2 possible specialized. enzymes on which programs These mitosis are, of protein 1st, depends, the and 2nd, the synthesis of those enzymes that direct the specific activities typical of the tissue. It appears that, although cells are capable of undertaking these 2 types of syntheses, none are capable of undertaking them both simultaneously; the operation of one synthetic program always seems to exclude the operation of the other (see p. 1699). This is again reminiscent of t.he situation in microorganisms in However, if the chalones do form is made part between be emphasized that to for of the mechanism possible synthetic in some tissues the choice is not simply between 2 alternatives. In some tissues, such as striped muscles and nerves, there is evi dently no choice at all, and at all times the cells continue synthesis for function. In other tissues, such as epi dermis (44, 339, 347), the cells are at least potentially capable of more than 2 types of synthesis. The many varieties of epidermis can be classified into 3 major groups: surface epidermis, which produces the outer sheet of “soft― keratin; hair bulbs, which produce “hard― keratin; and sebaceous glands, which produce fatty sebum. In all normal situations the cells either undergo mitosis or pro duce their typical proteins, but in abnormal situations the various types (357). Thus, after the destruction of these epidermal formation after the of cells types are may found contribute of a new and entirely destruction cholanthrene of the to be interchangeable of the skin surface any cells towards the typical surface epidermis; sebaceous glands by methyl new glands may be produced from the cells of BULL0uGH—Mitotic and Functional the outer follicle sheath (357, 358) ; and in certain circum stances the surface epidermis may even give rise to new hair follicles, each of which will possess a sebaceous gland (40, 42, 52, 350). Thus it appears that at least some of the epidermal cells retain a certain residual versatility that can be exploited in an emergency. Other examples of this type of reaction are found in liver cells (308) and in melanoblasts, which do not synthe size melanin until they receive an appropriate stimulus (43). Indeed, as Willis (526) has emphasized, any study of pathologic histology soon indicates what the various cell types “canbe or do in all manner of abnormal environ ments . . . and shows that great transformations of cellular structure . . . are possible in most tissues.― It. has been suggested by Weiss (512, 513) that when a differentiated cell changes its activities in this way the phenomenon should be called modulation. In the case of epidermis it is clear that such modulation occurs only as the result. of some drastic change in the cellular envitonment., which must in turn cause a significant intracellular change. III. MITOTIC HOMEOSTASIS A. CONTROL IN ADULT TISSUES BY CHALONES It is evident that in any “typicaltissue― the alternative programs of protein synthesis for niitosis and for tissue function differ in at least 1 important respect. Any cell that begins to prepare for mitosis is thereby committed to complete the process, but any cell that is synthesizing the proteins needed for normal tissue function is commonly able to revert to a relatively nonfunctional state from which it can then proceed to mitosis. Such reversion is seen in most tissues after wounding, during regeneration, in tissue culture, and in neoplasia, and it follows that the state of functional activity typical of most differentiated tissues may be regarded as unstable. Indeed, the impli cation is that not only the functional state of a tissue but the whole structure of an adult mammal may need at all times to be actively maintained against the possibility of collapse into mitotic anarchy. By contrast, the process of recurrent cell growth and mitosis is evidently entirely stable, and both in vitro and in neoplasia, cell populations that are behaving in this way appear to be potentially immortal. Thus each of the tissues of an adult mammal seems to be in a state of equilibrium and all except the most. highly modified types of cells, to be poised between an unstable functional state and a stable routine of recurrent mitosis. It is obvious that an understanding of the nature of the mechanisms that determine and maintain the actual point of balance within each tissue is of the greatest possible theoretical amid practical importance. One common theory has been that tissue mitotic activity may be con trolled by a tissue mitotic inhibitor greater the tissue mass within in such a way that the the body space, the lower will be the rate of cell production (see, for instance, Refs. 259,348,377,378). The earliest indications that this concept may indeed be broadly correct came from observations on organ regener ation and in particular from studies of the regeneration of Homeostasis 1687 which contains much contradictory evidence, has been Fe viewed by, for instance, Swanmi (467, 468) and Bullough (73), amid it is also discussed below (see p. 1691). The 1st significant success in the extraction of tissue-specific mi totic inhibitors with the necessary characteristics appears to have been that of Saetren (425, 426), who studied niitotic control iii kidney and liver; the 2nd was that of Bullough and Laurence (86, 87), who studied epidermis and suggested that such inhibitors should be called chalones; and the 3rd was that of Rytomaa arid Kiviniemi (423), who have extracted a granulocytic chalone. The epidermal chalone is water soluble, and it can easily be extracted when the epidermis is macerated. It is not species specific, amid it is capable of depressing mitotic activity in mouse epidermis both in vivo and in vitro (87). The epidermal chalone will also depress mitotic activity in cornea, sebaceous glands, and eso@)hageal lining el)ithehum, but not in other tissues tested. Con versely, aqueous extracts of a variety of other tissues do not. lossess the power to depress epidernial mitotic ac tivit.y. More recently it has been shown (78) that the epidermal chalone is nondialyzable and that. it. is destroyed by boiling and precipitated by alcohol. It is unstable in water solution but stable after lyophilizatiomi. By fractional precipitation most. of the chalone present in the original solution has been recovered in the small ire cipitate obtained when the alcohol concentration is raised from 70% to 80%. An in vitro analysis of the mode of actiomi of the epi dermal chalone has demonstrated 1 particularly important point, namely that this chalone is unable to exert its full power as a mitotic inhibitor in the absence of adrenalin (75, 87), and conversely it can be shown that. adrenalin has no effect on the epidermal mitotic rate unless the epidermal chalone is present. This raises the whole question of the relation of the adrenal glands to mitotic activity, which it is convenient to consider before returning to the question of the chalones. B. THE ROLE OF THE ADRENALS 1. The diurnal initotic cycle.—This discovery of the role of the adrenal glands in the control of epidermal mitotic activity has provided an explanation for the old mystery of the diurnal mitotic rhythm, which is so prominent a feature in the epidermis (64, 73). It is now evident that when an animal rests or sleeps the rate of secretion of adrenalin falls, the power of the chalone is reduced, and the epidermal mitotic rate rises; conversely, when an animal wakes and beconies active the rate of adrenalin secretion rises, the power of the chalone is increased, and the epidermal mitotic rate falls (83). As would be ex pected, adrenalectomy destroys this diurnal rhythm and causes the epidernial mitotic rate to rise to a constantly high level (83). Diurnal variations in the rate of adrenalin secretion have been described in man (275), rats (161), and mice (284), and in all cases it is evident that the adrenalin concentration is greatly reduced during rest and sleep. The same is true of noradrenalin, but it is now clear that kidney and liverin adultmice and rats. This literature,this substance cannot act as an effective substitute for Cancer Research 1688 adrenalin in preventing epidernial mitotic activity.2 After adrenalectomy the marked rise in the epidermal mitotic rate is associated with a reduction to about 0. 1 of the normal adrenalin concentration, but there is little if any that Vol. 25, November no such diurnal tissues, including rhythms epidermis appear 1965 to exist in fetal (509). Although more evidence is needed, it nevertheless begins to appear that a diurnal rhythm in the rate of DNA change in the concentration of noradrenalin (162). The synthesis may be found in any tissue that, having only a question whether the hormones of the adrenal cortex may moderate mitotic rate, shows a diurnal rhythm in the also play some part in controlling the mitotic rate is dis nunibers of cells engaged in mitosis, and that in tissues such as duodenal mucosa, in which the mitotic rate is high, cussed in the next section. Although the diurnal mit.otic cycle has been analyzed imi there may be little if any sign of either rhythm. greatest detail in epidermis, it is evident that similar cycles occur iii many other tissues. When the mitotic behavior of a variety of tissues and organs is considered, it is found Finally, diurnal cycle iii agreement mitotic with the cycle is inversely in the rate of adrenalin suggestion related secretion, that the to the diurnal it is well known that the range extends from those like kidney and liver, which rarely show any niitotic activity, through those like ej)idermiS, which show moderate mitotic activity, to those like active hair roots, which show very high mnitotic activity. Reviewing this range, and setting aside any hormone-dependent tissues (see p. 1703), it is immediately obvious that a diurnal niit.otic rhythm is commomily, if not universally, present except. when the mitotic rate is that any stressful situation results in both increased ac tivity of the adrenal glands (16, 159, 161, 162, 221, 313) and decreased mitotic activity of the tissues. Such de creased mitotic activity has been described in epidermis (66, 77, 83), cornea (361), liver (307), and erythrocyte either 500) during excitement or pain ; and in all the tissues of young rats after general disturbance imiduced by repeated changes of boxes, diet, or companions (459). In juvenile rats stress by starvation has also been shown to depress very low or very high. In adult miianinials diurnal mitotic cycles, in which the highest rat.e of cell division develops during periods of rest or sleep, have been described in the epidermis of mice and rats (70, 73) and in that of men (433), in cornea (6, 197, 501), iii lens epitheliuni (428), in oral epitheliumu (6, 490), in esophagus (6, 64, 137), in stomach mucosa,3 in intestinal niucosa (6, 64, 197), in rectal mucosa,3 in pancreatic exocrine epit.helium (136, 331), in epididymis3 (64), and possibly iii erythrocyte production (223). In addition, in tissues that are mitotically inert in normal adult mammals, typical diurnal mitotic cycles may de velop during regeneration after partial hepat.ectomy (21, 266, 287, 421); in mammary gland during pregnancy (319); and iii liver (60, 136), kidney (6, 136), adrenal cortex (138), outer orbital gland (136), and epiphyseal cartilage (447, 448) during the juvenile growth 1)eliod. In tissues like active hair roots, which show an extremely high niitotic rate, no diurnal rhythm is present. ; in tissues like those of the adult liver and kidney, which show an extremely low mitotic rate, no diurnal rhythm is de tect.able, but this may be due to the inadequacy of the usual technics. Of considerable theoretical importance are the recent demonstrations that the diurnal mitot.ic rhythm is not confined to the visible stages affects the preceding of mitosis but that phase of DNA synthesis. has described rhythmic changes undergoing DNA synthesis and in the numbers also in the rate it also Alov (6) of cells of RNA synthesis in cornea, tongue, liver, and lymphocytes, and he has indicated that both these rhythms coincide with the mitotic rhythm ; he failed to find any similar phenomenon in intestinal mucosa, which is a highly active tissue in which the mitotic rhythm is poorly defined. The existence of a diurnal cycle in DNA synthesis has also been found in ej)idermis and tongue by Oehlert and Block (375), in cornea by Tchoumak (480), and in epidermis, tongue, esophagus, and forestomach, but not in duodenal mucosa, production (51) during stress by starvation ; in epidermis during prolonged exercise (65, 83) ; in cornea during pro longed swimming S. Bullough and E. 3 M. J. Coleman, personal B. Laurence, communication. unpublished (68) and cornea (174, DNA synthesis in epithelium of tongue, forestomach, and hindstomach, amid in liver, pancreas, and adrenal gland (286). 2. A drenalin and the glucocorlicoid horrnones.—Turning next to the adrenal hormones themselves, it shown, both in vivo and in vitro, that adrenalin potent epidermal niitotic inhibitor 114), that the glucocorticoid exerting an anti-mitotic known hormones action if they has been is the most (68, 71, 83, 87, are also capable of are given in suffi ciently large doses (68, 482), and that the mineralocorticoid hormones are without any apparent effect (68). Besides epidermis, adrenalin has been shown to depress mitotic activity in cornea (48, 174), in lens epithelium,4 and in intestinal mucosa its high mitotic (158). In this rate the depression small and short lived, and in active last caused hair bulbs, tissue with is relatively which have perhaps the highest mitotic rate shown by any adult tissue, adrenalin has no effect at all even when it is injected in near-lethal doses.2 This last observation is of critical importance in that it indicates clearly that, by itself, adrenalin is not a mitotic inhibitor. The fact that its inhibitory action on mitosis is obviously tissue specific may be taken as indicating that before adrenalin can act as an inhibitor it must either combine or otherwise co operate with some substance present in the tissue. On the basis of the evidence obtained suggested that this substance from epidermis, is the tissue-specific it is chalone. Regarding the glucocorticoid hormones, it is generally believed that their secretion rate is directly related to the secretion rate of ACTH from the anterior pituitary gland, and it is also known that stressful conditions lead both to a higher rate of ACTH secretion (281, 387, 427) and to an increased blood flow through the adrenal gland (230). The rate of ACTH secretion is increased by the most by Pilgrimetal.(388). Itisinteresting, however,to note diverse 2 W. (6) ; in epiderniis types of stress—chemical, results. 4 M. Towlson, personal communication. environmental, and BuLL0uGH—Mitotic and Functional psychologic—and the implication is that the various types act on the anterior pituitary gland through a common nervous pathway, which l)OsSiblY includes the hypo thalamus (see Refs. 229 and 427). In such circumstances it is not surprising to discover that the secretion rate of the glucocorticoid hormones shows a pronounced diurnal rhythm (160, 209, 216, 222, 424, 497, 498) ; in addition it has been found that the reactivity of the adrenal cortex to ACTH is higher when an aninial is awake (182). The details of the diurnal changes are complex and more difficult to interpret. than those of the adrenalin rhythm. However, the evidence is such as to support the possibility that the glucocorticoid hormones may play some role in mitotic control. If they act similarly to adrenalin then 2 things should follow : 1st, they should inhibit mitosis selectively in the tissues that normally show a diurnal mitotic rhythm; and 2nd, when the stimulus of ACTH is removed by hypophysectomy there should be a rise in the mitot.ic rate of these same tissues. The published evidence is unfortunately not extensive, but on the 1st point it is already known that cortisol, or hydrocortisone, does inhibit mitosis in epidermis (68, 115, 258, 482), gastric mucosa (406), and regenerating liver (60) ; all these tissues show a diurnal mitotic rhythm. Conversely, it is known that cortisol has little or no effect on mitosis in duodenal crypts, intestinal lymph nodes (406, 413), and active hair bulbs (352) ; these tissues show little if any diurnal mitotic rhythm. Thus it is already obvious that, as with adrenalin, the action of the gluco corticoids is not mitosis specific but tissue specific. Again the implication may be that a tissue-specific cofactor, such as a chalone, may be needed before the anti-mitotic action can develop, but the information available is still insuffi cient to indicate whether the chalone-adrenalin inhibitory complex also normally contains a glucocorticoid element. In passing it is interesting to note that interference with the adrenal mechanism in embryos, whether by cortisol in jection or by adrenalectomy, may cause serious growth abnormalities (see Refs. 245, 345, 346). Regarding the effects of hypophysectomy, this operation is so drastic that it is not surprising to find conflicting evidence. However, although the disordered metabolism that follows removal of the pituitary has been found to depress mitosis in regenerating liver (60), there is strong evidence that this operation can result in a dramatic increase (145, in the 147, 481), mitotic rate of epidermis, and stomach mucosa sebaceous glands (30), all 3 of which normally show a diurnal mitotic cycle. Conversely, hy pophysectomy has been found to cause no change in the mitotic activity of the duodenal crypts (405), which normally show only slight signs of a diurnal mitotic cycle. It seems probable that in an operation of this magnitude the results obtained may be greatly affected by the technic used. It is unfortunate that no study of the effect of hypophys ectomy on the diurnal niitotic cycle appears to have been made with mammals. However, with amphibians, in which hypophysectomy is an easier operation, Scheving and Chiakulas (434) have found that in the corneal epi thelium of salamander tadpoles, not only is the over-all mitotic Homeostasis rate increased, 1689 but the diurnal niitot.ic rhythm is eliminated. 3. Conc1u@sions.—The evidence concerning diurnal mi totic rhythms, the anti-mit.ot.ic effects of stress, amid the actions of adrenalin arid other adrenal hormones has been listed at some length, partly because so much of it has not previously been extracted from the large Russian literature and partly because it illustrates so clearly that. close simi larities may exist between the mitot.ic control niechanisms of different tissues. In particular it shows that the type of mitotic control mechanism described for the epidermis by Bullough amid Laurence (83, 87) is unlikely to be unique. Since it is now evident that in ei)idermis the hour-by-hour variations in the mitotic rate are inversely related to the hour-by-hour variations iii the concentration of the chalone-adrenalin inhibitor within the cells, it may be presumed that similar chalones, which show similar relations with adrenalin, may exist. in other tissues that show a diurnal niitotic rhythm, arid perhaps even in all the tissues of the body. It follows that, after allowance is made for the effects of the varying adrenalin concentrations, the degree of mnitotic activity typical of any tissue may be explicable either in terms of the chalone concentration within the cells or of the degree of stability of the chalone-adrenalin complex. Thus, a tissue with a naturally low mit.otic rate may have either a high chalone cell content or a chalone with a high affinity for adrenalin; a tissue with a naturally high mit.otic rate dition. In both these changes in the adrenalin to exert much may show the opposite con extreme situations the diurnal concentration would be unlikely effect. A possibility exists that. the glucocorticoid hormones may also be important factors in the mitotic control mechanism. C. In considering attention THE WOUND REACTION the is confined reaction to the of tissues changes to wounding, iii the mitotic rate. Other aspects of wound healing, including the iniportamit question of cell migration, have been reviewed by, for instance, Giliman and Penn (189), Johnson and McMinn (273), Slome (453), and Abercrombie (2). The account given above of the recognition and ex traction of the epidermal of a study of the chalone mitotic was itself reactions of the the outconie cells closely ad jacent to an epidermal wound (81, 84). It is well known that such cells may develop a mitotic rate niore than 10 times the normal maximum rate seen during sleep, and for a long time it has been commonly held that this is the consequence of the diffusion outwards from the wound of a mitosis-stimulating “woundhormone.― This old theory was critically examined by Bullough and Laurence (81), who showed that the mitotic reaction in the neighborhood of an epidermal wound is in fact due not to the presence of a mitotic stimulant but to the temporary absence of a mitotic inhibitor. By means of differential wounding they also showed that the epidermiial mitotic inhibitor is tissue specific and that at least 2 other tissue-specific mitotic inhibitors also exist in mouse skin—i each in the hypodermis and the hair roots (82). A.s regards el)idermlS, it now appears that the mitotic inhibitor is the epidermal chalone, amid it follows that a hypodermal chalone amid a hair root chalone (see p. 1686) may also exist. If the high mitotic activity associated with local damage is due to a reduction in the chalomie concentration, then as the mitot.ic rate rises the mitoses should show an increasing independence of the inhibiting action of adrenalin, and when the mitot.ic activity reaches its niaximum they should be unaffected by adrenalin and should show no diurnal rhythm. It has been found that this is in fact the case, although it is possible that in epidermis the niitotic activity never achieves coniplet.e independence. The situation may be similar to that in the duodenal mucosa, which may respond to au adrenalin injection by a small and short-lived mitotic depression (158) and may show slight signs of a diurnal mitotic rhythm (64). Epidermal cells adjacent to a wound may therefore always retain a small quantity of chalone with which adrenalin can react. It may be noted that the mit.otic activity adjacent to an epidermal wound niay riot reniaimi unifornily high from hour to hour as has commonly been believed, but that it may proceed in a series of waves that have nothing in coniniori with a diurnal rhythm (235). This phenomenon has been clearly demonstrated by Harding and Srinivasan (226), who have found waves of DNA synthesis passing outwards from the edge of a corneal wound at an aj)proxi mate rate of l7Whr. Hell and Cruickshank (235) suggest that this phenomenon is the outcome of an induced tempo rary synchrony in the cell cycle, amid since it may be sug gested that a normal function of the chalone-adrenalin coniplex is to inhibit the duplication of DNA, the sudden withdrawal of the chalone would be expected to have this effect. The failure of adrenalin to liniit the high mitotic rate associated importance damage since with wounds is a point of considerable practical since it is obviously essential that any tissue should be repaired it is evident that, as quickly in addition as possible, to any normal and stress, the presence of a wound may itself be a stressful factor. Iii this connection it is intemesting to find that from about 1 to 8 hr after the infliction of a skin wound a high concen tration of monoaniirie oxidase develops around the cut (402, 457), arid it is possible that, by destroying adrenalin at art unusually rapid rate, this may help to stimulate the preparations for mitosis during the period before the chalone concentration is significantly reduced. It niust be eniphasized that the pattern of the mitotic reaction seen after mechanical injury is essentially similar to Vol. 25, November Cancer Research 1690 that seen after damage by chemicals, radiation, or a variety of parasites (see Refs. 73, 277). After any kind of injury the affected cells, if they are not too heavily damaged, will themselves react by mitosis ; if they are too heavily damaged, of if they are killed or removed alto gether, then it is the closely adjacent cells that react. In either case the reaction could be due to the failure of the damaged cells to synthesize adequate amounts of their characteristic chalone or to an unusually rapid diffusion of the chalone away from the damaged area [it is known that the water relations of the cells are severely disturbed (see Ref. 492)1, or to these 2 factors combined. Even in normal epidermis there is clearly a steady loss of chalone 1965 into the dermis and the blood, amid it follows that it must be constantly synthesized within the cells (81). It is important to note that the mitotic respomise to daniage in the epidermis does not begin until after a delay of about 24—36 hr. However, a much earlier reaction, beginning in about 3 hr, leads to a great increase in the RNA content of the damaged cells (492, 494, 495, 523—25), and in about 12—24hr there is a considerable increase in the DNA content (226, 235). The increase in the RNA content may be related to an increased rate of protein synthesis as the cells begin to alter their pattern of activity, perhaps especially in preparation for niitosis, while the increase in the DNA content is undoubtedly due to chromosonie duplication in preparation for mitosis. It may also be noted that considerable changes occur in the concentration and distribution of various enzymes as shown by histochemical methods (see Refs. 401—4), but the significance of these changes is not yet understood. Although the details of the wound reactions are best known in the case of the epidermis, it is important to emphasize that both the premitot.ic and the niitot.ic re actions follow an essentially similar pattern in all tissues that are capable of mitotic activity and that have been studied from this point of view. This has been confirmed, for instance, in hair follicles (17, 82), tongue (45), esoph agus (342), hypodermis (82), gall bladder and urinary bladder (340, 341), gastric mucosa (255), liver (100, 101, 330), these uterus tissues (508), amid mammary gland (368). the mitotic reaction to local damage In all is itself strictly local, and as in epidermis (45, 81), it comiimonly takes the form of a gradient with the highest mitotic activity close to the wound edge. However, it is obvious that in different tissues the time takemi for the full de velopment of this reaction may vary widely; in epidermis, for instance, the response is relatively rapid, while in hypodermis it is relatively slow (82). It is possible that such differences may reflect the differing times required for different cell types to change fiom one program of synthesis to another, and they may also be at least partly dependent on the times taken for the local chalone concen trations to fall to a low enough level. However, the most important single point emerging from a survey of the mitotic reactions shown by a variety of tissues to damage of any kind is that they are all essentially similar. It may therefore be concluded that the results obtained from an analysis of the reactions of any one tissue, such as wounded epidermis, may apply equally to most, if not all, other tissues that are capable of a mitotic reaction. In particular, since in the case of damaged epidermis the evidence now available favors the theory that the high mitotic activity is due to a reduced concentration of the epidermal chalone, it may follow that essentially similar chalone mechanisms may also be present in a wide variety of other tissues. D. REGENERATION 1 . Local and general milotic reactions.—It is evident that the effect of a small wound can only be to damage or destroy a minute proportion of the total cell mass of the tissue, and that the consequent local reduction in the chalone concentration is unlikely to have any significant general effect on the chalone concentration in the tissue BuLL0uGH—Mitotic and Functional as a whole. This may be why all attempts to show that the presence of 1 wound can affect the process of healing of a 2nd woumid made at a distance have always failed (1, 42). Since diffuses chalone highest it is known that the epidermal chalone normally into the adjacent tissues, it appears that each may show a pattern of distribution in which the concentration is in the tissue of origin, a lower concentration is in the intercellular fluid, and the lowest concentration is at the point or points where the chalone is either degraded or eliminated. This iniphes a steady synthesis within each tissue, a steady rate of diffusion from that tissue, and a relatively short life for each chalone molecule. Thus in any normal tissue there may exist a state of balance in which the rates of chalone production, diffusion, arid loss are adjusted so that the chalone concen tration within the cells is adequate to maintain the mitotic rate typical of that tissue. It is obvious that such a system could be disturbed either locally, owing to a reduced chalone concentration within a small area, or generally throughout the whole tissue, owing either to a generally reduced rate of chalone production or to a generally increased rate of chalone loss. A local disturbance would, of course, be the result expected of a wound, while a general disturbance would be expected to follow extensive tissue damage or destruction or re moval. Thus in the case of the liver a small wound leads only to a local mitotic reaction, as mentioned above, but when about 10 % of the liver is removed a general niit.otic response is seen throughout the whole organ, and when from 10 % to about 70 % is removed the mitotic that develops is in direct proportion tissue excised (see Refs. 60, 192). results have been obtained reaction to the amount. of Essentially similar by measuring the rate of DNA synthesis; not until some 9—12% of the liver is removed does a general response develop, and beyond this threshold the number of cells that synthesize DNA is directly pro portional to the amount of liver removed (62, 325). The reactions of the liver remnant after partial hepa tectomy have been closely studied, especially by histo chemical technics (see Ref. 60). These details are largely but in stressing the essential similarity between the general regeneration action and the local wound reaction it is important irrelevant to the present re to note that the nature argument, and sequence are closely similar in both the rate of RNA synthesis after partial hepatectomy wounded epidermis (494) ; is seen from about 12 hr extensive literature of the cellular reactions cases. In particular, a rise in becomes obvious within 6 hr (232, 436) and within 3 hr in an increased synthesis of DNA both in regenerating liver (for see Ref. 60) and in wounded epidermis (226, 235) ; mitotic activity rises to a high level in 24—30hr in regenerating liver (59, 109, 110, 228) and in 24—36hr in wounded epidermis (81) ; and there are many other simi larities. It must be noted that the figures given here for regenerating liver refer to the parenchymal parenchymal cells have a reaction 1 day (212, 228). Similarly cells ; the non that is slower by about in wounded skin the reaction of the hypodermal cells is slower than that of the epidermal cells (82). Descriptions of the cellular changes during kidney regeneration are less detailed, but these changes are also Homeostasis 1691 evidently similar (108, 143, 144, 150, 163, 276) ; indeed in all cases of wounding or of regeneration, the essential cell reaction is a temporary change from synthesis for tissue function to synthesis for mitosis. It, is now clear that neither in the liver (see Ref. 60) nor in the kidney (204, 449, 514) can this change be due to an increased functional load or to an increased blood supply. Also, since the degree of the mitotic response is in direct relation to the amount of tissue removed, it is clear that it is related to an absence of tissue arid riot., for instance, to the amount of tissue damaged. In both organs it is gener ally believed that some humoral agent is the controlling factor, though opinion is sharply divided as to whether this agent is a mitotic stimulant (see especially Ref. 380) or a mitotic inhibitor (see especially Ref. 73) produced by the tissue or organ involved. Indeed it is often con sidered that. both mitotic stimulants arid mitotic inhibitors may be involved (see Refs. 60, 366, 510), arid it has also been suggested that regenerative growth may be under the control of hormones produced by some endocrine gland arid transported to the reacting tissue in the blood (see Ref. 1). The most important single piece of evidence in favor of the humoral nature of the controlling agent conies from experiments with parabiosis in rats. fri such experiments it is found that partial hepatect.omy in one partner is followed by a regenerative response in the livers of both partners, amidit is therefore most probable that the message passing from the operated to the unoperated rat niust. be carried in the blood (61, 116, 256, 515). The few reported failures to obtain such responses may be explicable in terms of technic (60). It niay be added that essentially similar reactions have recently been obtained with liver autogralts in rats, in terms of both mitosis (503) arid DNA synthesis (310). 2. Hormones and regeneralion.—The suggestion that regenerative growth by mitosis may be under the control of “orthodox―horniones has been considered, for in stance, by Abercronnbie (1) arid Weinibren (510). In par' ticular, attempts have been made to demonstrate sonic influence on liver regeneration by the hormones of the anterior pituitary gland (106, 173, 243). In brief, it has been found that, although hypophysectomy may result in a reduced liver size (391), the effects of the pituitary hormones on the regenerative process are not clearly de fined. Weinbren (510) has concluded that any effects seen are more likely to “reflect. the changes in general met abolisnri in hypophysectomized animals rather than any interference with the basic restorative process,― and similarly, in relation to normal rej)lacement growth, Bullough (73) has concluded that “mostof the pituitary hormones, as well as such other hormones as thyroxin and insulin, are important to mitotic activity only in so far as they ensure the balanced working of the general metabolic complex.― There is, of course, a special group of tissues and organs that are under the direct control of particular niitogenic hormones (see p. 1703), but with these exceptions, and also with the exception of certain adrenal horniories, there is at present no indication that any of the orthodox hormones plays a direct part in the regenerative response. The growing belief is that those humoral agents that are Cancer Research 1692 evidently involved in the control of the regenerative re sponse will prove to be organ or tissue specific, to be synthesized in the organs or tissues on which they act, arid therefore to be internal secretions that cannot be classed with the orthodox hormones. It is the thesis of this review that these agents may be chalones that act as tissue-specific mitotic inhibitors in the manmier of a negative feedback mechanism. 3. Chalones and regeneration.—If this theory is correct then it should be possible to explain mitotic homeostasis in organs such as kidney or liver in the following manner. When the mitotic activity of the component tissues is low, this is because of the high chalone concentration existing within the cells. This high concentration is maintained by a rate of chalone production that is in balance with the rate of chalone loss, perhaps mainly into the blood. This rate of loss must be at least partly de pendent on the steepness of the diffusion gradient from the tissue to the blood, and therefore the lower the chalone concentration in the blood the greater will be the rate of loss. When a large part of an organ system such as the liver is removed, the blood chalone level must also fall, and this must result in an increased rate of chalone loss from the remaining part of the organ. that in this situation the survivimig cantly increase their rate of chalone lone concentration within these cells level at which mitotic activity is In such circumstances, the observed degree of tissue destruction On the assumption cells cannot signifi production, the cha may be reduced to a no longer inhibited. relation between the and the degree of the mitotic response, whether local or general, is readily understand able. Since it is also suggested that each tissue is controlled by its own specific chalone, it is obviously possible that the concentrations of the various chalones within the surviving part of an organ system may be reduced at different rates, or that different degrees of reduction may different tissues before the response In either case the differing mitotic reaction be needed times in develops. observed for instance in the tissues of the liver (3) would also be readily understandable. In this connection, it may be recalled that in regenerating liver, mitotic activity develops first in cells around the branches of the portal vein as though this is the area from which the chalones are first drained (227). The end of the regenerative response will, of course, come when the organ system is restored to its normal size in relation to the total body space, and when, in consequence, the normal chalone balance is re-established. Vol. 25, November who induced mitotic activity 1965 in miormal liver by diluting the blood, amid by Glinos (190, 191), who performed the converse experiment of inhibiting mitotic activity in regenerating liver by increasing the concentration of the blood, which he did by limiting the water intake. How ever, it must be noted, first, that in carefully controlled experiments involving massive changes in blood con centration Bucher (60) has found “noconsistent activa tion or suppression of regeneration,― and second, that increasing the concentration of the blood by limiting the water intake may result in a state of stress similar to that produced by limiting the food intake (83). The belief that stress may inhibit mitotic activity in regenerating liver is supported by Jaffe's of a diurnal mitotic rhythm. Contradictory of the literature particular, regeneration mitotic of the existence interpretations of results are a feature on liver and kidney regeneration. In while many authors have concluded is initiated by a reduced concentration inhibitor, controlled (266) report by an equal the number production of consider a mitotic that of a that it is stimulant. The literature has been extensively reviewed on many occasions (see especially Refs. 60, 73). Here it may be briefly noted that the serum of normal rats is reported to be capable of limiting mitotic activity in regenerating liver (261, 283, 461, 462, 466) ; that the serum of rats with regenerating livers may stimulate mitosis in the livers of normal rats (177, 249, 254, 299, 301, 455, 536) ; that the serum of hepatectomized rats may produce a greater than usual mitotic rate in livers of other hepatectomized rats (4, 445, 455, 461, 462); and that the dilution of serum, for instance with saline, is said to be able to induce mitosis in normal liver (60, 195, 365). However, in none of these experiments has it been critically established whether the results obtained are explicable in terms of a mitotic inhibitor or of a mitotic stimulant. It may be suggested that part at least of the confusion may be due to the fact that the concentration of any humoral agent in the blood is likely to be relatively low and that, consequently, it might be more promising to investigate the actions of tissue macerates or extracts. There is a considerable literature derived from this type of experiment, but again the results recorded are con tradictory, some for instance indicating that normal liver macerates or extracts contain a mitotic inhibitor, some that they are inactive, and some that they contain a mitotic stimulant. Considering first containing a mitotic those extracts that are described as inhibitor, the earliest positive results 4. Theexperimentalevidence.—A considerableproportion appear to be those of Saetren (425, 426), who showed that of the recorded liver experimental and the kidney results relating can be interpreted to both the to fit a theory of this type, which is closely similar to that put forward by Glinos (192, 193). Most of the experimental work has when a kidney macerate is introduced into the body cavity after unilateral nephrectomy it is capable of inhibiting of liquid in the regenerative mitotic activity in the remaining kidney and, similarly, that when a liver macerate is used it inhibits mitotic activity in a regenerating liver fragment. Each body or injections of macerates or aqueous extracts of the of these organs in attempts to change involved either alterations concerned tion of the humoral in the volume the concentra agents. Considering first the question of the concentratiomi of a humoral agent in the blood, some of the most important pioneer work was performed by Glinos amid Gey (195), actions is described as organ specific, and macer ates of organs other than kidney and liver did not produce any similar effects. Saetren's work is almost unique in that he paid particular attention to dosage, and he noted that the most powerful inhibitions were obtained when the amount of macerate introduced was equivalent to BULL0uGH—Mitotic and Functional Homeostasis the amount active of organ removed. agents were heat He also noted that labile and nondialyzable the ; and in this respect they resemble the epidermal chalone (78). Support for these conclusions has been provided by Stich and Florian (462) and Molimard (353). It has also been noted by Saetren (425) that damaged liver contains no appreciable Stich erating liver. have and amount and Florian of the (462) that However, been recorded inhibitory directly (496), and contradictory by Blomqvist Tumanischvili agent by the same is true of regen who results (46), Paschkis consider that (380), maccrates or extracts of liver, whether normal or regenerating, contain some mitosis-stimulating agent. For a full survey of this literature (510), and Bucher Finally, mention Malmgren obtained see Paschkis (380), Weinbren (60). must be made of the suggestion by and Mills (329) that a mitotic stimulus may be only from a liver maccrate that has been heated. They believe that liver tissue may contain both a mitotic inhibitor and a mitotic stimulant and that in normal circumstances their actions cancel each other out. When, however, the inhibitor is destroyed, the stimulant is unmasked and is able to act. The only conclusion that can be drawn from all this literature is that, in the particular contexts of the various experiments, each and all of the results obtained may be correct and that therefore the conditions in which the experiments were carried out, and in which the observa tions were made, may be of critical importance in evalu ating the conclusions that have emerged. In particular a survey of the literature shows that in both the serum experiments arid the organ extract experiments little at tention has usually been paid to dosage, which commonly appears to have been very low ; or to the degree of stability or instability of the extracts; or to the age of the animals, which were commonly young rats that had not yet reached their may full size so that a varying degree of mitotic naturally have been present in the kidney activity arid the liver; or to the state of the reacting tissue, in which the mitotic rate may vary from hour to hour according to a diurnal rhythm (266) and from day to day during the process of regeneration ; or to the proportion of the organ system removed, which determines the magnitude of the mitotic response and for optimal experimental purposes should be only 50 % of the possible maximum ; or to the time lag allowed either after the operation or after the injection before the mitotic response was measured ; or in some cases to the adequacy of the criteria used in measuring the response; or to the manner in which the experimental manipulations may have affected the ani mals, especially in terms of the degree of stress imposed. These and other difficulties that stand in the way of the proper evaluation of the available evidence have been stressed by Weinbren and MacDonald et al. experiments involving more critical technics various contradictions. 5. Mitotic inhibition (510), Bullough (73), Bucher (60), (326), and it is obvious that further a more systematic approach and are now needed to resolve all the and slimulus.—One possible cx planation for the conflicting results that have been ob tamed emerges from the suggestion that both an inhibition 1693 and a stimulus may normally be produced after a maccrate injection and that which of these is recorded depends On the time observation. lag allowed between the Thus Weinbren (510), injection amid the using rats with regenerating livers, found a mitotic depression after 1 day and a mitotic stimulus after 2 days, while Wilson arid Leduc (527), using young and adult normal mice, found a mitotic depression during the 2nd arid 3rd days arid a mitotic stimulus during the 5th—8th days. The differ ences in the timing of these responses may reflect differ ences between normal arid regenerating livers, between the dosages used, and perhaps also between the species. If it is confirmed that the reaction to the injection of a tissue homogenate is a rapid mitotic depression followed by a later mitotic stimulus, then 3 obvious possibilities exist. First, the homogenate may contain both an in hibitor arid a stimulant (329), arid these 2 elements may act successively; 2nd, if the homogenate contains a mitosis inhibiting chalone, the initial depression it induces may be followed by a later compensating stimulus; and 3rd, if the tissue homogenate is injurious to the cells, or if it becomes injurious when it autolyzes in the body cavity, the initial mitotic depression may be due to tissue damage, which, through reduced chalone production, ultimately leads to an increased mitotic rate. Regarding the 1st possibility, little or nothing can be added to the evidence already given. However, regarding the 2nd possibility, it is known in the case of epidermis that the chalone-adrenalin complex, when fully active, imposes a complete inhibition at a point just prior to the onset of mitosis, although it evidently has no similar effect at any point in the long phase of DNA duplication (83, 88). After such an inhibition has been imposed for several hr abnormally large numbers of cells accumulate, all fully l)repared to enter niitosis together as soon as the effectiveness of the chalone-adrenalin inhibition is ye duced. Thus, if the mitotic counts are made a few hr after the experiment begins, a mitotic depression may be recorded ; whereas if the counts are made later, when the inhibition is losing its power, an apparent, but unreal, mitotic stimulus may be found. It must also be added that this double effect may result from the action of endogenous adrenalin if for any reason the experi mental treatment results in serious stress, but in this case the mitotic reactions should be equally obvious in all the tissues that normally show a diurnal mitotic cycle. The 3rd possibility—that the injected maccrate is injurious, or that it becomes so—raises again the old theory that the stimulus to regenerative growth by mitosis, which develops after tissue damage of any kind, is due to the production of “necrohormones―(see Refs. 290, 483). It seems reasonable to regard such substances, if indeed they can be proved to exist, as essentially the same as the hypothetical “woundhormones.― However, since Bullough and Laurence (81, 82) have provided evidence that “woundhormones― do not exist at all, some doubt as to the existence of “necrohormones―also arises. 6. The effects of metabolic damage.—It does, however, remain possible that tissue extracts may contain sub stances that might be derived, for instance, from the lysosomes (see Ref. 370), that would not normally be Cancer Research 1694 present free in the body, that are injurious to the cells in a specific (483) or nonspecific (527) manner, and that may not form any significant part of the basic mechanism of healing and regeneration. Such substances may act in the generally toxic manner of chloroform (438), trypan blue (386), or carbon t.et.rachloride (117, 309), all of which cause damage to the liver cells, as well as to certain other cells, amid consequently result in a raised mitotic rate. When such damaging agents are applied to mice clear signs of cell damage in the liver precede the mitotic re action (528), and similarly injections of liver homogenates have been described as causing “somedegree of necrosis― before the development of the mitotic response (15, 290). This (527), by a liver, l)Oirit was also considered by Wilson and Leduc who recorded an initial mitotic depression followed mitotic stimulus after injections of maccrates of kidney, and even boiled egg yolk and concluded that although “noinjury was evident in most of our experiments, it would be difficult to rule this possibility out completely.― With any cytotoxic agent the question of dose is obviously important.. If it is too high the damage may be too great, and indeed Wilson and Leduc (527) have imidicated that the higher the dose of liver maccrate the lower the ultimate mitotic stimulus. Ac cording to Bullough (73), the correct dose for the stimulation of mitotic activity can “bedefined as that which is sufficient to cause some cellular damage but not sufficient to damage the cells so seriously that they can rio longer divide.― Iii considering the wide variety of chemical and physical factors the application of which leads through tissue damage to increased mitot.ic activity, Bullough (73) has emphasized that the “commonfactor in all these seem ingly diverse situations is evidemitly a particular level of disturbance of, or damage to, some aspect of cell me tabolism― arid that in all probability “suchdamage results not in the production of a stimulating ‘woundhormone' but in the failure to produce some factor which had pre viously prevented the cells from indulging in their basic functions of growth arid mitosis.― An essentially similar conclusion has been expressed by Tsarievm (492, 494, 495), who has shown that when mouse skin is damaged by pressure in such a mariner that no cells are killed, and therefore no mitosis-stimulating ‘ ‘necrohormones― are produced, there is a sharp rise in epidermal mitotic ac tivity after about 24 hr. This agrees with the observa tion of Bullough and Laurence (81) that massage is all that is needed to disturb the metabolism of the cells and to raise the mitotic rate in ear epidermis. Other situations in which cell damage may lead to an increased mitotic rate are those in which ligatures have been applied or tension has been induced. Thus it is well known that a raised mitotic rate occurs in the kidney after the ligature of either the ureter (31, 239, 240) or the renal artery (144). If it is assumed that such treat ment results in a reduced rate of chalone production in the damaged kidney, then the rise in the mitotic rate seen both in that kidney and in its opposite partner is easily explicable. Some confirmation for this point of view comes from Saetren (425), who found that a kidney 5 R. Tsanev, personal communication. Vol. 25, November 1965 damaged for 10 days by ureter obstruction no longer contained any of the organ-specific mitotic inhibitor that he was able to extract from normal kidneys. In experi merits by Herlant (239, 240), the mitotic reaction of the damaged kidney lasted for about a week, when atrophy began, while the mitotic reaction of the opposite kidney continued for more than 4 weeks, showing the same level and duration as that seen after unilateral nephrec tomy. Similar results have been obtained in the bile duct system (102, 324, 511) and in the gall bladder (265) during distension caused by ligature, in the endometrium and myometrium during distension of the uterine lumen (412), and also most probably in the epidermis during tension (41). The mitotic reaction of the epidermis of limbs imiwhich the blood flow has been stopped by liga ture has been studied by Tsanev (493). This treatment so damages the cells that after the ligatures are removed high mitotic activity repeated applications develops over the whole area; of ligatures this high mitotic by ac tivity can be maintained for long periods. Tsanev (493) has also shown that after blood stasis a similar reaction develops in the ducts of the mammary glands. 7. The effectsof the adrenal horinones.—Inspite of all the contradictory conclusions that have been reached re garding mitotic control during regeneration, none of the available evidence directly contradicts the suggestion that such control may be exercised in terms of a chalone type of negative feedback mechanism. If this suggestion is correct, and if the chalones of the liver and kidney are similar to that of the epidermis, then the adrenal hormones should also be involved. The actions of these hormones may not be apparent in normal liver and kidney because the mitotic activity is negligible, but as the mitotic rate rises during regeneration they may become more obvious. In the 1st place the regenerating tissues may begin to show a diurnal mitotic rhythm, and in the case of the liver such a rhythm has indeed been described (23, 105, 266). Second, a mitotic depression should develop in regenerat ing tissues during stress and after adrenalin injections, but unfortunately such situations do not seem to have been studied. Third, adrenalectomy should lead to increased mitotic activity, and this has been described in normal liver (249), in regenerating liver (236, 237), and in compensatory renal hypertrophy (19). However, it must be added that this operation may sometimes lead to such a degree of metabolic disturbance, especially regarding the sodium balance, that mitotic inhibition may sometimes be seen (204). Finally, the glucocorticoid hormones may be expected to exert some influence, and it is now well known that these hormones do strongly inhibit mitotic activity in normal liver (249) and in regenerating liver (see Ref. 60). Hem ingway (238) has concluded that mitotic control in the liver is partly partly through exercised through plasma proteins and the corticosteroids that are bound to them, and this is in obvious ment. inhibit agreement with the present argu It is also known that glucocorticoid hormones DNA synthesis in regenerating liver (see Ref. 60), and Guzek (219) has suggested that the anti-mitotic actions of these substances are in fact the secondary conse BuLL0uGH—Mitotic quence of their “director indirect interference in DNA synthesis.― The question of the points of action of chalones, adrenalin, and glucocorticoid hormones is discussed below on p. 1710. 8. Other evidence on regeneration.—In conclusion 2 other points of interest may be mentioned. First., it is evident that the mitotic reaction to partial hepatectomy, including the DNA synthesis reaction, develops much more rapidly in young animals than it does in old animals (see Ref. 60). The livers of these young animals still normally show many @ mitoses and may therefore contain 1695 and Functional Homeostasis a relatively low chalone concentration. A similarly low chalone con centration may also exist in livers damaged, for in stance, by cirrhosis, and it is therefore interesting to note that the mitotic reaction to partial hepatectomy develops most rapidly in the livers that have suffered the greatest amount of such damage (400). Again this is in accord with Saetren's observation (425) that damaged liver contains less than the normal chalonie concentration. The 2nd point of interest is that after unilateral mie phrectomy in a pregnant rat, the regenerative rnitot.ic response is seen only in the remaining maternal kidney; there is no reaction in the fetal kidneys (203). It thus appears that maternal chalones are unable to pass the placenta, arid this would appear to be a necessary pre caution that prevents the maternal mitotic control mechanisms from interfering with fetal growth. There are also no diurnal rhythms in DNA synthesis in fetal tissues, including epidermis (509). 9. Conclusions.—In view of the conflicting evidence and the conflicting opinions it is particularly difficult to reach any general conclusions regarding the mechanisms under lying regenerative growth. It is clear that much of the confusion arises from inadequately planned experiments and especially from a general failure to study the mitotic reactions from hour to hour during a sufficiently long period of time. There is also the possibility that tissue cx tracts may contain not only the typical chalone but also certain toxic substances which, by damaging cells, niay in duce complex pathologic changes in the mitotic rate. However, in spite of these difficulties, the following points may be emphasized. First, it is evident that with increasing tissue damage the local mitotic reactions seen alongside wounds grade imperceptibly into the general mi totic reactions of regeneration ; there is such a close and detailed similarity between these local and general reac tions that it is reasonable to conclude that they must be essentially similar. Since the mitotic reaction alongside a wound is most readily explicable in terms of the chalone theory, and since the evidence concerning the mitotic re action in regeneration is not inconsistent with this theory, it is also reasonable to believe that regenerative growth may be basically explicable in terms of a negative feedback chalone mechanism. The 2nd point, which lends support to this conclusion, is the evident involvement of adrenalin and the glucocorticoid hormones in the control of re generative growth. series of phases of activity, amid it is of considerable impor tance that these should be adequately defined arid that. the point or points of action of the chalone-adrenalin complex should be determined. There is a large literature devoted to attempts to disentangle amiddefine these various phases (for early evidence see Ref. 252; for later evidence see Refs. 334—37) arid also to determine the times spent by different types of cells both in the whole niitotic cycle arid in each of the phases within the cycle (see Refs. 37, 132, 306). Technics for determining the duration of the miii totic cycle arid of its various parts are described by, for imistance, Fry et al. (178), Smith amid Dendy (454), Puck and Steffan (396), Quastler (399), and Hof and Ying (247). The 4 phases commonly recognized u.s constituting the mitotic cycle are: mitosis itself (also called “M―) ; the “1stgap―(or “G1―); the phase of DNA synthesis (or “8―); and the “2ndgap― (or “G2―), from which the cell passes into mitosis once more. However, with increased knowl edge this simple analysis of the mitotic cycle is no longer adequate, amid on present evidence it is possible to recog nize at least 6 phases, which were recently defined by Bullough (74). Still more recently, it has become possible to suggest slight modifications to these definitions to mdi cate more precisely what may be happening in each of the phases, and to relate them to that other important cell ac tivity, which is specialization for tissue function. The 6 phases, arranged in sequence, are : apopha.se (a new term defimied below) ; dichophase (from which a cell passes either to specialization for function or for mitosis) ; prospha.se, which is itself divisible into 3 phases (early I)rOsl)hase, phase of DNA synthesis, and anitephase) ; and mitosis itself (see Chart 1). Apophase : This is a term proposed here for the first. time (and derived from amro, which implies “moving away from―) to describe the period (commonly included iii G1) between the end of mitosis and the beginning of the dicho phase. Although this is a phase about which little is known, it is reasonable to suggest that the main preoccu pations of the cell at this time may be recovery from the previous mitosis and growth in bulk so that the cell re covers the size typical of its kind. Although it is well known that cell growth does not necessarily follow mitosis, it seems probable that it may commonly do so in the cells apophase dichophase I¼..@ I tissue function early prosphase DNA synthesis prosphose antephase ______ mitosis E. THE MITOTIC CYCLE 1 . The phases of the cycle.—It is well known that cell, in passing from one mitosis to the next, traverses any a CHART 1.—The phases of the cell cycle. From dichophase cell may specialize either for tissue function or for mitosis. the of adult mammalian somatic tissues. This phase may therefore be regarded as the final part of the mitotic cycle, in which the cell returns to its normal state. Dichophase : This is the period (commonly included in G1) during which the cell is committed either to specialize for tissue function or to specialize for mitosis (the term is derived from Sr@o,which expresses doubt between 2 ways ahead). It appears that the dichopha.se may sometimes be short but that commonly it may be protracted. Its significance is discussed on p. 1701. Prosphase : This is the period during which the cell com pletes all the necessary syntheses to enable mitosis to begin (the term is derived from @rpoa,which expresses movement towards). At the moment it can be subdivided into the early prosphase (commonly included in G1), the phase of DNA synthesis (or S), and the antephase (or G2). The most dramatic of these phases, and consequently the one that has been Vol. 25, November Cancer Research 1696 most intensively studied, is that of DNA synthesis. It is commonly forgotten that this cannot be the actual beginning of the preparation for mitosis but that at least 1 earlier phase must exist. 2. The initiation of the prosphase.—The prosphase may be defined as beginning with the synthesis of the 1st of the sequence of enzymes that underlie the mitotic process, and it is already known that accelerated RNA and protein syntheses do occur before the onset of the phase of DNA synthesis (250, 251, 257, 316, 322). It has been suggested that 2 enzymes in particular are likely to be of critical im port.ance at. this early stage. The 1st is that enizynie, or group of enzymes, which promotes histone synthesis; it is evident, for instance during liver regeneration, that the histone content of the nucleus begins to increase before the onset of DNA synthesis (217, 257). The 2nd is the DNA polymerase, without which the phase of DNA syn thesis could riot begin. Mazia (337) has questioned whether enzymes of these kinds may be synthesized di rectly on the chromosomes or whether, like other proteins, they are produced in the cytoplasm. He has also ques tioned whether the DNA polymerase alone is capable of inducing DNA synthesis arid has shown that another emizyme may also be needed to prime the DNA before it can respond. Busch et al. (99) have suggested that DNA may be inactive when combined with historic arid that the DNA strands are “releasedfor replication by the removal of specific histories protecting against their replication,― and it is possible that this removal may be the essence of the priming process. However, emphasis must be placed on the concluding words of Busch et al. (99) that “itis painfully apparent that we are just at the earliest stages of comprehension of the role of proteins in regulation of synthetic activities of the cell arid in mitosis.― 3. The phase of DNA synthesis.—It is significant that once the phase of DNA synthesis has begun it evidently always proceeds to completion. This is, of course, also true of the whole process—DNA synthesis-antephase mitosis—but it is riot yet certain whether the actual point of no return is at the beginning of the phase of DNA syn thesis or at sonic previous point in early prosphase. It follows that although active RNA and protein synthesis are necessary to initiate DNA replicatiomi, they may not be necessary to sustaimi it (225, 322). Indeed there is some 1965 evidence that during this phase the rate of RNA synthesis is normally depressed ; that it is not stopped entirely may be due to the fact that the DNA molecules do not appear to replicate simultaneously and that consequently at any given time a certain number are always mi a state in which they can initiate RNA synthesis (372, 394, 451). In microorganisms it has been suggested that the process of replication may jass in a linear manner along the entire genome (321), and in HeLa cells there is evidence that the average rate of DNA synthesis may reach its maximum at approximately the midpoint of the phase (485). It has also been postulated that, since the start of the phase of DNA synthesis may be dependent on the separation of the DNA from the histones, so also the end of the phase may involve their final recombination (217). 4. Antephase and mitosis.—The final period of prepara tion for mitosis is the antephase, which extends from the end of DNA synthesis to the beginning of mitosis. In dif ferent tissues and in different circumstances it has a dura tion of from about 1 to many hr, and with the chromo somal syntheses already completed, it appears to be the time when the final cytopla.smic syntheses are completed. Taylor (479) has shown that a partial inhibition of protein symithesis results in a prolonged antephase. Unfortunately relatively little is known of these final syntheses, but it has been suggested that they probably include the production of those molecules that, at the ap propriate moment, become reversibly aggregated to form the spindle apparatus (see Ref. 516). The possible bind ing mechanism used in the assembly of these macromolecu lar units has been discussed by 1\Iazia (333, 334). Another suggestion has been that the antephase is the time when an adequate store of energy-rich molecules is established to support the cell throughout mitosis. This suggestion has been widely accepted (70, 335, 467)—and indeed it has been shown that the spindle apparatus car ries an ATPase (336)—but recently some important con trary evidence has been published (see Ref. 337). The theory originally arose from a study of mouse epidermis (see Ref. 79) that showed that mitotic activity is depend emit on glucose arid oxygen, but that any cell entering mitosis becomes independent of these substances. It al SO became apparent that the actual point of inhibition caused by glucose or oxygen lack is towards the end of anitephase. Later, similar results were obtained with cleaving sea urchin eggs (see Ref. 467), but recently these results have been criticized by Epel (153), who has shown that these eggs at all times possess an energy store sufficient to sup POrt full activity for about 20 mm, by which time the ATP level falls to about 50 % of the normal. If at this moment the cells are part way through the process of mitosis, then they fail to complete the division. Doubt as to the estab lishment of an energy store has also been expressed by Amoore (8—13),who has studied pea root tips. In these he has shown that the effect of oxygen lack appears to be exercized through some interference with a “mitotic ferrous complex.― However, although these conclusions may be accepted, they do miot at the moment seem to apply to such adult mammalian tissues as epidermis, or even to embryonic tis BULL0uGH—Mitotic 1697 and Functional Homeostasis sues (128). In adult epidermis the blockage imposed by oxygen lack develops immediately in any cells in late ante phase, but it does not develop in any cells actually in mitosis, even though this process may last for several hr (88). Thus, in the case of epidermis, no evidence exists to contradict the conclusion that the antephase may be a time when the energy debts of the dividing cell are paid in advance. However, this is certainly a matter that needs further investigation ; the results of the experiments of Gelfant (184), which may appear to be relevant, have al ready been criticized (85) and need not be discussed here. It remains to consider the manner in which a cell passes from antephase into mitosis. It is possible that this happens simply as the natural consequence of the comple tion of all the necessary preparations, but it is also possi ble, as suggested, for instance, by i\Iazia (335) and Taylor (479), that the transition to mitosis may be trig gered by the synthesis of some particular enzyme. These suggestions are hypothetical, and the question remains unanswered. The activities of a cell in mitosis are dramatic arid in dude especially the assembly of the spindle apparatus, the separation and movement apart of the chromosomes, and the production of new cell walls. It is remarkable how little general agreement exists as to the manner in which any of these tasks is accomplished (see especially Refs. 335—37). However, there does appear to be general agree ment that during mitosis both RNA amid protein synthesis are depressed and even arrested (285, 393), and it is be lieved that this may be the consequemice of the condensa tion of the chromosomes, the breakdown of the nuclear membrane, the disappearance of the nucleolus, and the disruption of the endopla.smic reticulum. It is evidently because of this dismantling of the cell machinery that all preparations for mitosis must be completed in advance and that, once a mitosis has begun, it is so immune to outside interference as to give the impression of being ami all-or none reaction (see Refs. 70, 73). 5. RNA and protein synthesis.—From this survey the suggestion emerges that the essential RNA arid protein syntheses of the mitotic cycle may take place in a sequence of periods of high and low activity. Setting aside the phase of general protein synthesis that may commonly characterize the apophase and that may be regarded merely as a final phase of recovery from the previous divi sion, the following periods can be recognized : a phase of protein synthesis, which initiates preparations for the next mitosis (early prosphase) ; a phase during which the chro mosomes, being concerned with DNA synthesis, are not in a state to induce active protein synthesis (phase of DNA synthesis) ; a 2nd phase of protein synthesis in preparation for mitosis (antephase) ; and finally the phase of mitosis itself, during which protein synthesis is again depressed. known that the phases of both DNA synthesis It seems probable that if the early prosphase and the ante phase of DNA synthesis and that there also appears to be an accumulation of cells, in dichophase or early prosphase, that enter the phase of DNA synthesis in abnormally large numbers when food is again provided (104, 286). To this may be added the obvious evidemice that after wounding or during regeneration, when it is suggested that the chalone concentration falls, unusually large numbers of cells enter the phase of DNA synthesis. The implica phase are the times when protein synthesis is most active, then they may also be the times when metabolic inhibitors are able to exert their maximum effects and in particular when any natural mitotic homeostatic mechanism, acting through an inhibition of the syntheses that precede mi tosis, may be expected to operate most powerfully. In agreement with these conclusions it is already well show a high degree of independence and mitosis once they have begun and that the 2 points in the mitotic cycle that are most sensitive to interference are located before the onset of DNA synthesis arid before the onset of mitosis. Thus Mazia (337) has comicluded that no case is known mi which DNA synthesis fails to be completed once it has begun, and Bullough (70) has reached a similar conclusion re garding mitosis itself. By contrast it is well known that cells may be arrested for days or months or years before they enter the l)hase of DNA synthesis, amid Bullough and Laurence (83), by strengthemiing the chalone-adrenalin inhibitor, have shown that cells in antephase may be pre vented from entering mitosis for periods of at least 2 days. 6. The action of the chalone complex.—This raises the question of the l)Oinit or points in the mitotic cycle at which the chalone-adrenalin complex exerts its inhibitory action. It is at once clear that any interference in apophase is most improbable. If apophase can be partly defined as a period of general cell growth, and if the chalone complex sup presses the special syntheses needed for mitosis, then there is little reason to suspect any action at this time. The first l)Ossible points of action of the chalone complex may be the dichophase, the transition from dichophase to early prosphase, arid the early prosphase. Although no ade quate investigation has yet been made, certain evidence is already available. Thus Bullough arid Rytomaa (90) have shown that the granulocytic chalone, iroduced by mature granulocytes, reduces the numbers of bone marrow myelo cytes that enter the phase of DNA duplication, while Brown et al. (57) have shown that in a stressful situation, which may be presumed to involve the production of cx cessive amounts of adrenalin, there is also a reduction in the numbers of intestinial cells entering the phase of DNA duplication. It has been shown above that stress and adrenalini provide the explanation for the diurnal mitotic cycle, arid it is therefore significant that diurnal variations in the miumbers of cells commencing DNA synthesis have been described in a wide variety of mammalian tissues (388). It is alsointeresting that these variations are found only in tissues that also show a diurnal mitotic cycle; in tissues in which the mitotic rate is so high that no clear cycle is evident, there is also miodiurnal variation in the numbers of cells entering the phase of DNA symithesis. If these results are interpreted to mean that the chalone adrenalin complex controls entry into the Phase of DNA synthesis in the same way as it controls entry into mitosis, then another parallel may be expected. It is known that in a stressful situation, such as starvation, cells may be arrested in amitephase in such a way that large numbers accumulate and are ready to enter mitosis together as soon as the adrenalin concentration falls (83) ; it is now known that starvation reduces the numbers of cells entering the Cancer Research 1698 tion is that these cells had previously been prevented from taking this step by the inhibitory action of the chalone, and it follows that all the available evidence combines to support the suggestion that the chalone-adrenalin complex acts to inhibit the entry of a cell into the prosphase. Comisidering next the phase of DNA synthesis, the cvi dence of Pilgrim amid \Iaurer (389) suggests that there is no diurnal rhyt.hm in the duration of this phase. This suggestion in turn may be taken to indicate that adrenalin, amid therefore the chalone-adrenalin complex, has little if any effect on a cell once it has begun to synthesize DNA. This conclusion is strengthened by the observation of Bullough and Laurence (83) that, both in vivo and in vitro, a high concentration of adrenalin results in the accumula tion of abmiormally large numbers of cells in antephase. This can oniy mean that adrenalin does not prevent the cells that are synthesizing DNA from progressing into the antephase. However, while not contradicting this con elusion, it has been rioted by Rytomaa6 that not only does the granulocytic chalone reduce the number of cells en tering the phase of DNA synthesis, but that it also slows the passage of cells through this phase. This is a matter for further investigation but already it is clear that cells in the phase of DNA duplication are not readily suscepti ble to inhibition. In contrast, the evidence reviewed above points over whelmingly to the conclusion that the antephase is highly susceptible to the inhibitory action of the chalone adrenalin complex in a wide variety of tissues. This is shown by the existence of diurnal rhythms ; by the anti mitotic effects of stress in vivo, and of adrenalin and cha lone in vivo and in vitro ; and by the raised mitotic rate after adrenalectomy arid after chalonie reduction by wound ing. It is also interesting, and important to emphasize, that the main point of inhibition appears to be towards the end of antephiLSe, perhaps only a matter of minutes before the cells enter the earliest recognizable stage of mitosis. Thus, in experiments in which epidermis containing no mi toses was taken from stressed mice and placed in a saline medium, high mitotic activity developed immediately (83). This may lend some support to the idea that miii tosis is initiated by some trigger synthesis (335, 479) that is particularly sensitive to inhibition. As regards mitosis itself, it has been found in epidermis that the chalone complex can act to slow the process but not to stop it and, conversely, that in the close neighbor hood of wounds, where the chalone concentration is cvi dently reduced, the cells pass through mitosis more quickly than usual (88) . However, when the situation is studied more closely it is found that the chalone complex Vol. 25, November that the progress of a cell through enced only by earlier events. 1965 mitosis is usually influ F. CONCLUSIONS The evidence reviewed above suggests that mitotic homeostasis in the tissues in an adult mammal may corn rnonly depend on the anti-mitotic actions of tissue-specific chalones and that these actions are strengthened by adrenalin and perhaps also by the glucocorticoid hor monies. It appears that the chalone may be synthesized at a constant rate within the cells on which it selectively acts, while the concentration of the adrenal hormones varies according to the degree of stress felt by the animal. It may be noted that, since the chalone mechanism evolved to suit conditions in wild animals, which may commonly be subject to considerable stress, mitotic horneostasis may operate at less than optimum efficiency in well-kept do mesticated animals. The diurnal mitotic cycle appears to depend on the in stability of a chalone-adrenalin complex, which evidently breaks down when the adrenalin concentration falls during rest or sleep. The details of this cycle are now seen to be more complex than was originally thought. When the adrenalin concentration rises after a period of sleep, cells are inhibited from entering the prosphase, but those cells that are already in the prosphase continue their prepara tions until they are arrested in the antephase ; the few that manage to enter mitosis pass through it relatively slowly. When the adrenalin concentration falls during rest or sleep, cells enter freely into both the prosphase and mi tosis. The cells that enter mitosis are a mixed group, being partly those that are then completing the prosphase and partly those that have accumulated in antephase dur ing the previous waking hours. The cells that complete prosphase during sleep pass through mitosis relatively quickly, but those that had previously been held in ante phase pass through mitosis even more quickly. The result is that the beginning of a sleep period is characterized by a particularly high rate of mitosis; later in the sleep period the mitotic rate falls to a lower level that is determined by the rate at which cells are then completing the prosphase (see Chart 2). In considering the different types of diurnal mitotic cycles seen in the various tissues it is evident that they can be arranged to show every gradation between 2 cx tremes. These extremes are illustrated, on the one hand, by tissues such as those of the kidney and liver, in which has little or no action on a cell once it has entered mitosis ; the slower passage through mitosis is the result of the action of the cOmj)leX in antephase. It is interesting to note that the slower passage through mitosis that results from a lack of oxygen or glucose is also partly or wholly due to an inhibi tion in antephase, and it may be recalled that colchicine blocks mitosis in metaphase only as a result of its previous actiomi on the cell in antephase. Although the full signifi canice of these observations is unknown, it does appear CHART 2.—Diagram illustrating a typical diurnal mitotic cycle. The highest mitotic activity is at the beginnin'@ of the sleep 6 T. Rytomaa, personal communication. period (from Coleman'). BuLL0uGH—Mitotic and Functional @ the mitotic rate is so low that the counting technics now in use are inadequate to determine whether or riot a diurnal cycle exists, and on the other hand, by tissues such as that of active hair root, in which the mitotic rate remains con stantly at the maximum. In an idealized form the range of types of diurnal rhythms lying between these extremes is illustrated in Chart 3, and it appears logical to suggest that the mitotic rate, which determines the type of cycle seen, may be in inverse proportion to the chalone content of the tissue. The evidence also suggests that when, after tissue damage, the chalone concentration is reduced amid the mitotic rate rises, the type of diurnal cycle also changes and that in extreme cases, when the mitotic rate rises to a maximum, the diurnal cycle disappears. It has been stressed that when the chalonie concentration falls after tissue damage there is a collapse of functional specialization amid a reversion to specialization for mitosis, and the hypothesis has been advanced that the choice be tween specialization for function and specialization for mitosis is normally made in terms of the chalone conicen tration within the cells. If this is true, then a chalone may be regarded as acting on the genome in the manner in which an effector is believed to act in a microorganism. This hypothesis differs fundamentally from that of Weiss (514), which explains tissue growth in terms of tissue-specific ‘ ‘templates'‘amid ‘ ‘anti-templates. ‘ ‘The “templates―are regarded as catalysts in the production of that specialized “diversity of compounds― that is typical of the particular cell type, while the “anti-templates,― which diffuse throughout the body and have a certain superficial resemblance to the chalones, act by inhibiting the “templates.― This hypothesis implies that when, within the cells of a tissue, there is active synthesis of the specialized tissue proteins, then mitosis leading to tssue growth naturally follows, whereas in fact it is evident that. when a cell indulges in synthesis for tissue function, this automatically involves an inhibition of synthesis for nuito sis. Homeostasis Iv. MITOTIC 1699 AND FUNCTIONAL A. THE BASIC MECHANmsM 1 . Mitosis and cell function.—If the chalone-adrenalin complex acts in the manner of an effector to determine which region of the facultative genome shall be active at any given moment, then when the efficiency of the cha Ionic complex is high cells may tend to prepare for tissue function, while when the efficiency of the chalonie complex is low they may tend to prepare for mitosis. Thus the primary function of the chalone complex may be the Pro motion arid maintenance tioni of niitosis, by which of tissue function, arid the inhibi the existence of chalones has been recognized, may be merely the secondary outeome of this. There may even be a hidden diurnal rhythm in the number of cells preparing for tissue function, and if @oit would of course be the reverse of the diurnal mitotic rhythm. It has often been suggested that mitotic activity amid functiomial activity are mutually exclusive. Thus; con sidering normal epidermis, it could be suggested that mi totic activity is the exclusive function of the unspecialized cells of the basal layer, and that once a cell lia.s moved into the more superficial layers arid has commenced keratin synthesis, mitosis becomes impossible. However, when this example is examined in greater detail, it is found that the basal cells of the germinative layer already contain bundles of fine fibrils, which are regarded as the precursors of keratin (54, 347, 374, 442). In fact the cells are clearly recognizable as epidermal cells, and indeed mitotic activity may commonly occur, as in human epidermis, in the basal layer of the stratum spinosum in which the fibrils are even more conspicuous. Evidence that. cells showing clear signs of their special nature arid function may also indulge in mitotic activity has often been given ; examples of this are seen durimig endochondral osteogenesis (535), eryt.hrocyte production (373), and granulocyte production (381). However, in all cases of this kind it is clear that It is also difficult to perceive any relationships between cell specialization the chalones, which are regarded as tissue-specific inhibi tors, and such substances as “retine,― which has been de scribed by Szent-Gyorgyi and his colleagues as a nonspe cific inhibitor (233, 470—72). HOMEOSTASIS beyomid a certain stage of mitosis no longer occurs. Mäkeläamid Nossal (328), studying the transformation of lymphocyte to plasma cell, noted an inverse relation between the anti body-forming and the DNA-synthesizing capacity of the cell, and Wessels (517) has made the important point that “thefact that mitotic figures are occasionally found in cells in the early stages of specific synthesis may be less significant. . . . . than the fact that rio mitotic figures are seen in the more fully differentiated cells.― It seems that this comment, made especially in relation to pancreas acinar cells, is generally true of all cell types, arid as Weiss (513) has said, “thegeneral impairment of proliferative capacity iii cells undergoing ascribed to the fact that niitotic prerequisites, entiation products.― terminal specialization most of their substance, is diverted can be including to the building of differ It thus appears that mitotic activity arid specific cell sleep period CHART 3.—The hour-by-hour mitotic tissues; for explanation see the text. activity of a variety of function are indeed mutually exclusive but that mitosis only ceases beyond a certain point in the range of increas ing preparation for tissue function. It is obvious that a graded effect of this type could be related to the effects of a steadily increasing concentration of the chalone complex within the cell, arid certainly the evidence does not run Cancer Research 1700 Vol. 25, November counter to the suggestion that mitotic homeostasis and functional homeostasis may be achieved by one and the same control mechanism. The maintenance of any tissue in a functional state automatically involves the control of its mitotic activity. In this connection it is interesting to note that when cer taini tissues are incubated in vitro the cells tend to lose their specialization for function and to prepare for mitosis. This collapse of organization is readily explicable if it is postulated that a small fragment of tissue immersed in a relatively large volume of nutrient medium suffers such a marked reduction in the intracellular chalone concentra tion that the cells revert to mitotic activity. It is also relevant to note that when, as a result of this activity, a large enough cell mass is built up, the cells limit their prep arationis for mitosis arid recommence their specialized syntheses. This has been described in vitro in mouse fibro blasts, which, when their numbers approach a critical level, begin to produce collagen (210, 211), and in pan cress acinar cells, which in similar circumstances begin to produce zymogeni (517, 518). This latter case is especially interesting since it is the innermost cells that first cease mitotic activity and commence zymogeni production ; the peripheral cells, which might be expected to possess a lower chalone content, continue to undergo mitosis (see also Ref.364). One problem in such in vitro reactions is the source, if any, of the adrenalin cofactor. No information on this point is available, but there is evidences that a chalone may itself possess considerable activity which, in vivo, is merely augmented by the adrenalin. When small specialized, most groups nondividing probable that of already specialized, or partly cells are placed in vitro, it seems the process by which they reacquire a mitotic ability must be the same process by which similar cells in vivo reacquire a mitotic ability after wounding or during regeneration. It is this kind of evidence that serves to stress the normal instability of the specialized tissue functions arid also to emphasize that functional homeostasis is merely the obverse of mitotic homeostasis. However, it is also obvious that in many, if not all, functional tissues there are cells that have entirely lost any capacity for mitosis. It seems improbable that the epi dermal cells of the stratum granulosum, or those granulo cytes that have been released from the bone marrow, can ever divide again. It is obvious that this must be true of the nonnucleated erythrocytes, amid it is also obvious that this phase must be the final one in the life of a cell and that it can end only in death. It is evident that the pro portion of the cell population in this final phase varies from tissue to tissue: it is relatively high in the granulocyte sys tem and relatively low in the epidermis. In the nervous system amid in striped muscle, all cells might be considered to be in this phase. It appears that in any typical tissue there exist at least 4 main categories of cells (see Chart 4) : the progenitor cells (P) that are involved in mitotic cycles ; the immature cells (I) that have ceased, or are ceasing, to divide, amidare pre paring for tissue function ; the mature cells (M) that may or may not be functional and that, if circumstances die tate, are capable of reversion to mitosis ; and the mature 1965 D CHART 4.—Diagram illustrating the relations between the different cell types in a typical tissue. P, progenitor cells; I, immature cells; M, mature cells; D, mature cells moving towards death. For explanation see the text. cells (D) that are perhaps always functional but cannot revert to mitosis arid are approaching death. It must be emphasized that the boundaries between the 4 cell cate gories are probably riot as sharply defined as they appear in Chart 4 and that in fact each category may merge im perceptibly into the next. Epidermis is an unusual tissue in which these cell categories are stratified and in which a 5th category of cells—dead ones—are retained as the stratum corneum. In most other tissues the various cell types seem to be mixed indiscriminately. 2. Life expectancy of cells.—It is also important to note that the life expectancies of the 4 categories of cells are quite different. Cells of type P form a potentially irn mortal population, as is shown by their behavior in vitro and in neoplasia. Cells entering I and reaching M thereby acquire a certain limited life expectancy since M normally leads only to D and to death, and this life expectancy is evidently typical of the tissue to which they belong. However, in unusual circumstances involving a fall in the chalone concentration or, in certain tissues, a rise in the concentration of some specific mitogenic hormone, the cells in I and M can return to P and thus apparently become rejuvenated. Cells in D can only go forward to death, and their life expectancy may perhaps prove to be rela tively constant in any one tissue. in considering the balamice within a tissue between the processes of cell production, cell function, and cell death it is obviously inadequate to consider only mitosis and function. The life expectancy of the cells entering I and M arid the consequent rate of death from D are also criti cally important factors. It must be concluded that in any adult tissue not only is the average rate of cell gain by mi tosis in exact balance with the average rate of cell loss by death, but the proportion of the cell population that is pre paring to be or actually is functional (I, M, and D) is de pendent on the life expectancy of the cells entering I. Since the chalone complex appears to play an important role in the maintenance of tissue homeosta,sis it is interest ing to consider what influence, if any, it may have on the life expectancy of the cells in I, 111,and D and thus on the rate of cell death. From many examples of tissues with widely differing mitotic rates it is possible to suggest that, as in duodenal mucosa, a low chalone concentration may lead to a high mitotic rate combined with a short life ex pectancy in I, M, and D, while a high chalone concentra tion, like that in the liver, may lead to a low mitotic rate combined with a long life expectancy, especially perhaps in M. It thus becomes important chalone mechanism to consider whether the may act not only to promote the func BuLL0UGH—Mitotic tional maturity of the cells but also to prolong their life expectancy. In this connection it may be recalled that when, by long-continued stress induced by partial starva tion, Bullough and Ebling (76) maintained the epidermal mitotic rate of adult male mice at a quarter of its normal level for 4 weeks, no change was recorded in either epi dermal thickness or sebaceous gland size. It was obvious that in these 2 tissues the rate of cell loss must have been reduced to a quarter of its normal level. The converse situation has been described by van Scott and Ekel (439), who have shown that with increased epidermal mitosis in psoriasis the life expectancy of the cells was reduced from the normal 27 days to only 4 days, and by Skjaeggestad (452), who, from a study of the epidermis of hairless mice, has concluded that all hyperplasia-producing agents also induce an increased rate of cell loss. Similarly in the rat liver MacDonald (323) has described how normal cells divide at intervals of from 191 to 453 days, while with a raised mitotic rate in cirrhosis the cells have a life span of only about 26 days. The conclusions must be that when the efficiency of the chalone complex is decreased, the raised mitotic rate is matched by a shorter life expectancy and therefore a higher rate of cell death, and that when the efficiency of the complex is increased, the reduced mitotic rate is matched by a longer life expectancy and therefore a lower rate of cell death. It must, however, be emphasized that only within limits is a change in the mitotic rate exactly matched by an op posite change in the life expectancy of the functional cells. In epidermis Bullough and Laurence2 have noted that when, in middle age, the number of mitoses increases to about 3 times the normal figure the thickness of the epi dermis remains unchanged, while van Scott and Ekel (439) have noted that when, in psoriasis, the number of mitoses increases to almost 30 times the normal figure the epi dermis becomes thickened. In this latter case it is evident that the decrease in the cell life-span was inadequate to offset completely the increase in the mitotic rate, and the tissue increased in size until it became stabilized at a new point of balance. Thus, @ the evidence suggests that the horneostatic 1701 and Functional Homeostasis media nism of which the chalone complex forms an important part controls the mitotic rate, the degree of synthesis for tissue function, and the length of life of the cells in I, M, and D, and the question arises how one mechanism could exert such a variety of actions. The simplest suggestion is that they all may be the outcome of the one basic cia lone action, which is to promote the active synthesis of those proteins on which the specialized functions of the cell depend—an action that not only involves blocking any alternative types of synthesis, but also maintains the func tional efficiency of the cells at a higher level for a longer period. This hypothesis is illustrated in Chart 5. The examples shown are taken from mouse data and include the duodenal mucosa, in which the mitotic rate is high and the life expectancy of the cells in M and D is about 2 days (311) ; the ear epidermis, in which the mitotic rate is moderate and the life expectancy of the cells in ill and D is about 25 days (439, 443) ; and the liver, in which the mitotic rate is low and the life expectancy of the cells in M and D is about 3000 days (323). These are all, of LiHweak hchalon @HIJ H@ ‘ chalone action—,—— .moderate @!I'@M JiJ strong chalone action I' LMI CHART 5.—Diagrams illustrating cell types in different tissues. the different P, progenitor proportions of cells; I, immature cells; M, mature cells; D, mature cells moving towards death. Top, duodenal mucosa with a high rate of cell production and loss; middle, epidermis with a moderate rate of cell production and loss; and bottom, liver with a low rate of cell production and loss. course, normal amidstable situations; but if, by tissue dam age leading to a lowered chalone concentration, the mitotic rate rises in either the epidermis or the liver, then it may be expected that temporarily these tissues will move closer to the condition shown by the duodenal mucosa. If this argument is taken to its theoretical limits then at one extreme, with no chalone present at all, M and D would disappear and all cells would remain in P; sonic such situation may perhaps be found in cases of advanced malig nancy. At the other extreme, with an excess of the cha lone complex, all cells would enter and remain in Al, and their life span would be infinite; in effect this may be the case in the mouse liver, in which the estimated cell life span of 3000 days greatly exceeds the possible life-span of the animal. It is, of course, obviously important that the life expectancy of the cells in Al should be directly related to the degree of efficiency of the chalone mechanism. If this were not so then any tissue in which mitotic activity declined and finally ceased would be in danger of total diasppearance as its cells passed steadily through M to D and death. 3. The nature of the dichophase.—Ithas been suggested above that the decision whether to specialize for mitosis or for tissue function is taken by a cell in dichophase iii terms of the intracellular chalone concentration. How ever, it is evident that while in some tissues this decision may be taken promptly, in others the cells may remain in a state of indecision for a long time. Tissues in which the decision is taken promptly are those with a high mitotic rate. An example is an ac tively growing hair root, the cells of which appear to lack any effective chalone control and therefore to pass almost directly from apophase into prosphase. The speed at which this occurs, together with the shorter duration of Cancer Research 1702 prosj)ha.se amid mitosis (88), results in recurrent mitotic cycles, each of which is completed in the shortest time needed for the essential symitheses. In an adult mam malian tissue it does not seem possible to reduce the duration of the mitotic cycle to less than about 12 hr (80, Vol. 25, November 1965 chance and may vary widely between cells, but in any particular tissue the average length of time a cell remains in dichophase may be precisely definable. The variable effects of chance on individual (Yells are shown when a cell population is artificially synchronized so that the 440). cells leave mitosis As the effectiveness of the chalonie control increases in a tissue the duration of the whole mitotic cycle also increases, arid it has been shown by Bullough amid Laurence (88) that this is at least partly due to the increased time spent by the cells in l)rosPhase arid mitosis. However, it is well known that the duration of the phase of DNA syn thesis is not likely to exceed about 10 or 12 hr (see Refs. desymichronization occurs in the l)art of the cell cycle that may now be recognized as the dichophase (485). When the chalonie concentration falls after any type of cell damage, not only do the cells in dichophase tend to move towards the prosphase, but the functional cells in M 103, 291, 312), the amitephase about commencing DNA symithesis in as little as 4 hr (235); functional liver acinar cells, after hepatectomy, pass back through dichophase to commence DNA synthesis in about 20 hr (see Ref. 60). Thus on the present hypothesis the dichophase emerges about. the maximum remain awake time that 16 hr [which is an animal may normally (see Ref. 83)], and mitosis about 6 hr [which is about the longest time taken by an epidermal mitosis with excess adrenalin (see Ref. 88)]; and although little is known of the length of the apophase, it is possible that this too may only last for a matter of hours. Thus even in extreme conditions the total duration of the whole sequence, l)rosphasc-mitosis-apophase, seems unlikely to exceedsome 2 or 3 days. However, it is well known that in tissues with moderate or low mitotic rates a mitotic cycle may take very much longer than this (see Refs. 37, 306) ; examples are the cells of the basal epidermal layer, which undergo mitosis about once in 25 days (439, 443) and the cells of rat liver, which may undergo mitosis only once in about 200—400 days (323). It therefore appears probable that in such tissues the cells must spend a considerable cisively in the dichophase. time resting mdc It seems probable that any cell entering the dichophase is exposed to conflicting urges towards mitosis and to wards functional maturity; this is expressed diagram matically in Chart 6. With a low chalone concentration the decision to enter mit.osis again would be quickly taken, in unison. It is found also tend to move back into the cells already in dichophase may as perhaps the most critical that progressive prosphase. Epidermal react to wounding by of all the phases through which a cell passes and also as a phase in which, if the chalone concentration is at sonic indeterminate level, a cell may remain indecisively poised between the 2 possibilities that are open to it. 4. Conclusions.—In any stable adult tissue the balance between cell gain and cell loss appears to be determined, 1st, by the characteristic chalone concentration, and 2nd, by the characteristic life expectancy of the maturing cells. The primary function of a chalone may be to promote maturity, and since it is commonly believed that chalones are produced mainly by mature cells (73, 260, 348), chalone production may be self-promoting. The characteristic life expectancy of the maturing cells varies, perhaps widely, among different tissues, but within any one tissue it changes inversely with the mi totic rate. It is important to note that when such a change takes place it appears to apply equally to the new cells then being produced amid to the already mature cells. the dichophase would be very short, and few if any cells would enter I, M, amid D; with a high chalone concentra tion the dichophase would be equally short, the cells would rapidly enter ill and become functional, and P would be depleted and even eliminated; but with an inter mediate chalone concentration the dichophase might be very long and might consequently contain a large number of cells. The actual length of time that any particular cell remains in dichophase appears to be a matter of @ M 1' . \7@dichophase @apophase ,mitosis @@%%% prosphose CHART 6.—Diagram phase. illustrating the significance of the log. cell life span dicho Cells entering this phase may remain there for an mdc terminate period or tissue function. before moving towards either the prosphase I, immature cells; M, mature cells; D, mature cells moving towards death. CHART production 7.—The inverse relationship between the rate of cell and the length of life of the mature cells of the seba ceous gland [taken from Ebling (146, 147)1. BuLL0uGH—Mitotic and Functional It is also probable that the mitotic rate amidthe life expect ancy of the mature ship (see Chart cells have an inverse log-log relation 7). The result is that beyond a certain 1703 Homeostas'is siderable informat.iomi already exists regarding thyro tropini (1, 429). It is, however, surprising how little of the vast literature that has been devoted to these sub point the increasing mitotic rate outpaces the increasing ccli death rate so that the tissue increases in mass until it reaches a new point of balance. However, in order to obtain a small increase in tissue mass it is necessary that there be a great increase in the mitotic rate. stances is concerned with the rnitot.ic response of the tar get tissues, arid this is especially true in the case of the androgemis, which are normally vaguely described as stimiiu latinig the “growth― of the male accessory sexual organs It follows that the proportion of tissue mass to total dent that such growth does involve a raised mnitot.ic rate and also that the mitogenic stimulus of the androgens is body mass tends to remain remarkably constant., amid in (see, for instance, Refs. 376, 395). It is, however, cvi deed it seems possible that tissue mass itself might be de steadily termined by the combined actions of the rate of chalone production and the life expectancy of the mature cells. Thus it might be suggested that a tissue of large mass and productive by studies terone on such nonsexual tissues as the epidermis low mitotic rate may be characterized by a relatively low arid the sebaceous (146, 360). rate of chalone production and a long cell life ; a tissue of small mass and low mitotic rate, by a relatively high rate of chalone production and a long cell life ; a tissue of large mass and high mitotic rate, by a relatively low rate of chalone production and a short cell life; amid a tissue of small mass and high mitotic rate, by a relatively high rate of chalone production arid a short cell life. It is also evident that the mitotic stimulus exerted by the estrogens, which is felt es@)ecially in the female acces sory sexual tissues but also in other nionsexual tissues, must be steadily maintained. However, when the stimulus is first applied it results not iii a constantly elevated mitotic rate but in a series of sharply defined waves of mitotic activity (see Refs. 63, 154, 157). The reaction is complex, arid it also differs in its timing in dif fererit tissues. Thus the lining epithelia of vagina arid uterus show their earliest reaction in about 12 hr (39, 385) and reach maximum mitotic activity in from 24 to 36 hr, while the lining epithelium of the Fallopian tube amid the B. THE VERSATILITYOF THE MECHANmSM 1 . Methods of alteration of tis&ue mass.—It is evident that the point of balance between cell gain arid cell loss, which may also determine the tissue mass, is normally remarkably stable. Only after wounding or during re generation, when the mitotic rate is greatly increased, is this normal balance disturbed. However, the disturb ance is temporary and self-cancelling, and as the chalonie concentration returns to normal the original I)Oinit of balance between cell gain and cell loss amid the original tissue mass are both regained. Only if cell damage is constantly inflicted, for instance by any form of tissue irritant, can a new point tissue of increased size. However, of balance be established in a certain tissues and organs must be able read ily to increase their mass in response to particular needs. Thus the tissues of the accessory sexual organs become enlarged during the breeding season in response to the action of certain mitogenic hormones, and the mass of the erythrocytic tissue is increased during oxygen lack in response to erythropoietin. From such examples the impression is gained that the mitogenic hormones amid the poietins may constitute a 2nd order of mitotic and func tional control imposed on the basic chalone control mecha nisms of the target tissues. 2. The mitogenic hormones.—It is well known that a number of hormones can exert a profound effect on the mitotic activity of certain so-called target tissues. It must be emphasized that the present argument is con cerned only with those hormones that, in physiologic con centrations, exert a direct effect on the mitotic rate. Other hormones, not considered here, may be important to mitosis but only in so far as they ensure the balanced working of the general metabolic complex; in a normal adult mammal these are always present in adequate con centrations. The best known examples of mitogenic hormones are the androgens and estrogens, although con smooth maintained throughout activity. of the steady muscle the whole This conclusion glands mitotic stimulus cells of the uterus period of re has been confirmed may exerted take by testos (89, 359) 2—3days to reach maximum mitotic activity (63). Similarly, in a normal estrous cycle the vaginal amid uterine epit.helia reach their 1st mitotic peak in late diestrus, while the other 2 tissues do riot do so until estrus (38, 63, 154, 155). It is also obvious that, at the peak of the mitotic reaction, those tissues that react the fastest contain the highest proportion of cells in mitosis. This situation is reminiscent of the different reaction times of the tissues of the skin after wounding (81, 82), and indeed there are obvious similarities between tissue reactions to a reduced chalone concentration and tissue reactions to an increased mitogenic hornione concentra tion. After the 1st mitotic reaction to the estrogenic stimulus there is usually a period of at least a day during which the mitotic rate falls to a low level (63, 154, 155). Then if the estrogen concentration remains high, this is followed by a 2nd wave of mitotic activity, which is commonly smaller thami the 1st wave. After this the waves are damped down and the mitotic rate settles at a moderate level, which however is comisiderably higher than that of the ovariectomized controls (383). A full explanation of this phemiornenionis not yet avail able, but Epifanova (156) has argued that the high imiitial mitotic stimulus may be reduced because of an estrogen induced rise in the adrenalin amid glucocorticoid hornionie content of the blood. It is well known that estrus is a time of stress when the adrenal glands increase consider ably in size (69) and when unusually large amounts of adrenalin are absorbed by the uterus (530, 531). How ever, it has been shown that, in mice stressed for long periods by starvation, “theuterus responded normally to physiological amounts of estradiol― (49), arid this sug Cancer Research 1704 gests that the stimulus of an estrogen may be more powerful than the inhibition of the adrenal hormones. As an alternative explanation it may be suggested that the mitotic waves are closely similar in form to those which occur in the epidermis alongside wounds (226, 235) and which have been plausibly explained in terms of an imposed mitotic synchrony. It seems that any type of sudden stimulus to mitotic activity may result mi an unu sually large number of cells entering prosphase and mitosis approximately in unison, arid if the stimulus continues the cells may also enter a 2nd mitotic cycle while still preserving some of their synchrony. The evident similarities between wound reactions and estrogen-induced reactions help to suggest a possible relationship between the chalones and the mitogenic hormones. Since chalones are regarded as tissue specific arid since mitogemiic hormomies tend to be target-tissue specific, the simplest explanation would be to suggest that each mitogenic hormone selectively neutralizes the chalories of its target tissues. In this connection Jensen (271) has already remarked that “itis curious that so little attention has been given to the possibility that estrogen may inhibit some growth-restraining factor, for many aspects of its action are more in keeping with an inhibitory rather than a stimulating role― arid that the key property of anìy estrogen “mightwell be an ability to associate with sonic cellular factor and inhibit its func tion.― The result of such an action would be a reduction in the effective chalone concentrations in the target tis sues, arid if this interpretation is correct then certain con sequences should follow. In the 1st place, while the mitotic rate of the vaginal arid uterine epithelia in the ovariectomized animal may be too low to show any diurnal rhythm, as the effective chalonie concentration is reduced by the rise in concen trationi of the est.rogenic hormone, a diurnal rhythm may appear [compare the situation in regenerating liver (266, 421)]. That this is indeed so is indicated by Bertalanify arid Lau (38), who have shown that diurnal mitotic cycles arc evident in most l)hases of the estrous cycle in the vaginal arid uterine epithelia. In the vagina the diurnal cycle disappears when mitotic activity is at its maximum, as it. is also known to do in epidermis during the j)eriOd of mnaximnum reaction to a wound, arid in both cases this could indicate a severe reduction in the effective chalone conicenitration. Innthe 2nd place, if a hormone-induced mit.ot.ic reaction is basically similar to a wound reaction, then in threshold or subthrcshold situations the 2 should be additive. In this connection it has recently been shown by Prop7 that, when mammary gland tissue is maintained in vitro, mitotic activity may indeed be stimulated when a subthreshold hormone stimulus is combined with a subthreshold wound stimulus. This is an important point on which further evidence is needed. With a normal wound in a hormone dependent tissue the mit.otic reaction is so great that the hormone is unable to induce the development of airy more mitoses (for example, see Refs. 63, 368), and indeed in a full wound reaction there should be little or no chalone left for the hormone to neutralize. 7 F. J. A. Prop, personal communication. Vol. 25, November 1965 In the 3rd place, if a mitogenic hormone normally acts by neutralizing a chalone, then the increased mitotic rate should result in a shorter dichophase and in a shorter ex pectation of life in the cells in the functional phase. Re garding the 1st point, Epifanova (156) has noted that estrogenic treatment may cause a 7-fold increase mi the numbers of mitoses seen and a 7-fold decrease in the length of the interphase, and Peckham and Kiekhofcr (383) have shown that the whole mitotic cycle may be completed in about 13 hr. Regarding the 2nd point, in an ovarec tomized animal the rate of mitotic activity in the vaginal lining is very low and the life expectancy of the mature cells is very long; after treatment with estrogen the life span of the miondividing cells is reduced to between 30 and 45 hr (383). Even more remarkable figures demonstrat ing an inverse log-log relationship between hormone dependent mitotic activity amid the life expectancy of the nondividing cells are given by Ebling (146, 147) in rela tion to the sebaceous glands of normal, castrated, hypo physectomized and testosterone-treated adult rats (see Chart 7). These results are particularly important since they emphasize that although a target tissue, and the organ of which it forms a part, may become larger in size when stimulated by a mitogenic hormone, they do not become as large as would be expected from a consideration of the mitotic rate alone. It is, of course, obvious that mitogenic hormones in duce a variety of cell reactions besides the increased mi totic rate; in particular, the new cells that are produced begin to function in a new way (see Refs. 151, 271). For example, under sex hormone influence, considerable cyto plasmic changes occur in the uterine cells (180), the vagi nal epithelium produces keratin and thus becomes corni fled (97), the cells of the male accessory glands secrete the seminal fluid (395), and in the thyroid the pituitary thyrotropic hormone also stimulates the synthesis and release of the thyroid hormone (198). Such reactions involve the synthesis of unusual quantities of messenger RNA (107, 200, 282), which leads to the active synthesis of new proteins both fri accessory sexual tissues (502) and in nonisexual tissues (435, 441). It appears that when a target tissue is unstimulated and the mitotic rate is negligible the majority of the cells, which have an indefinitely long life-span, are mature but nonfunctional. They may be regarded as belonging to type M, shown in Chart 8. The hormonal stimulus causes them to revert to type P and thus to begin mitosis. Many of the newly formed cells then pass to maturity again, but now because of a severely reduced life expect ancy they pass from type ill, which is nonfunctional, to type D, which is functional. Thus they are able to func tion actively in the mariner typical of the tissue in the hours or days or weeks that remain before they die. The sequence of this reaction is shown in Chart 8. When the hormone is withdrawn and the chalone con centration is again high the mitotically active type P cells are transformed to type Al and the functional type D cells must go forward to death, with the result that the tissue shrinks in mass. 3. The poietins.—\Iost of the dispersed cells of the BuLL0uGH—Mitotic ±lt CHART8.—The sequence of events in a target tissue when it is stimulated by a mitogenic hormone. The mature cells (M) are nonfunctional, and the consequence of the production of mitoti cally active progenitor cells (P) is the production of mature functional cells (D) that are moving towards death. body fall into 3 groups—the granulocytes, eryt.hrocytes, and lymphocytes—and the manner in which these are produced is of particular interest. The gramiulocytes arc the end product of the process of granulopoiesis, which is generally believed to be stimulated amid con trolled by a substance called granulopoictin, erythrocytes are the product stimulated by erythropoietin. while the of erythropoiesis, which is The more complex prob 1cm of the lymphocytes is dealt with in the next section. The mechanisms by which control is exercised over the processes of cell production and cell loss in the granulo cytic and erythrocytic systems are evidently complex, and they are not yet fully understood. Regarding the granulocytic system, Patt amid Maloney (381) have stated that “littleis known about its beginnings and endings, its dimensions and functions, and even less about its controls.― In this system most of the available iniforma tion refers to the neutrophils, which are polymorpho nuclear leukocytes. These are considered to origimiate from a population of stem cells, which give rise in the boric marrow to a sequence of cell types—the mycloblasts, promyelocytes, and myclocytes—that show increasing degrees of maturation but also undergo mitosis (90). Finally, they give rise to the metamyclocytes, which show no mitosis and mature into granulocytes. These are stored mi the bone marrow until they are released for a limited life in the blood and the tissues (sec Ref. 122). The situation in the erythrocytic similar system is essentially (see Ref. 292), and the cells arc usually considered to originate from the same stem cells as those that give rise to the granulocytes. In the bone marrow the matura tion of the erythrocytic progenitor cells proceeds through a sequence of cell types—the pronormoblasts, basophilic normoblasts, and polychromatic normoblasts—that show increasing degrees of maturation and that all undergo mitosis. Finally, into reticulocytes, the oldest cells become 1705 and Functional Homeostasis transformed which are incapable of mitosis and mature into erythrocytes. These are released into the blood immediately after they are formed, and they then have only a limited life-span. Described in this way, the granulocytic amid crythro cytic systems each constitute a typical tissue system, like that for instance of the epidermis, in that they con tam cells that are mitotically active, cells that show in creasing maturation accompanied by decreasing niit.otic activity, and mature functional cells with a limited life expectancy. However, they differ markedly from a nor ma! tissue in that they evidently share the same group of mitotically active stem cells, which must therefore be considered to be pluripotential. Although the emphasis in the literature has been mainly on the l)ossible mariner of control of these 2 systems by their respective poictins, in the i)resenit context it is mieces sary to consider whether each may contain a typical chalomie mechaniisni. Since in both systems the mature cells are dispersed it would, of course, be necessary to suppose that any chalone produced by them would have to lass via the blood stream to reach arid influence the dividing arid maturing cells in the boric marrow. It would also be expected, since in both systems the rate of ccli production is very high, that the chalone concentra tion in the blood may be low. In such circumstances a reduction in the already low chalone content of the blood might have relatively slight stiniulatory effect, but an increase either in the chalone comicentrationi or in the ac tivity of the adrenals might have a marked inhibitory effect. As regards the gramiulocyte tissue, the evidence in favor of the existence of a negative feedback control of the chalorie type has been assembled by Osgood (377, 378). In particular it has been shown that a decrease in leukocyte numbers leads to increased granulocyte production (see Ref. 122) amid that an increase in leuko cyte numbers inhibits granulocyte production, that fresh blood contains some factor that inhibits granulocyte production while old blood does not (520), amid also that fresh serum inhibits mitosis in bone marrow in vitro (423). From evidence of this kind and from a study of leukemia, Osgood (377, 378) has proposed a theory of niitotic comi trol in the granulocyte system amid in tissues in general that is fundamentally similar to the chalomic theory out lined above. However, in addition to the basic micgat.ive feedback mechanism, Osgood lists a long series of second ary mitotic comitrols, which includes vitamins, electrolytes, nutrients, temperature, CO2 temision, pH, and blood amid lymph flow. Such factors, if they influence mitosis at all, are most likely to do so in a nonspecific manmier arid are umilikely to form any significant part of a tissue-specific mechamiism. Some attempts have been made to extract a granulo cytic chalonc from mature gramiulocytea (122, 123, 423), and recently Rytomaa and Kiviniemi8 have obtained a substance having the required characteristics when tested on the mitotic rate of bone marrow cells in vitro. This substance is water soluble, unstable, nondialyzable, and relatively low in molecular weight, all of which charac teristics it shares with the epidermal chalone. S T. Rytomaa and K. Kiviniemi, personal communication. 1706 Cancer Research As regards the crythrocytic tissue, relatively little attention has been paid to the possible existence of an eryt.hrocytic chalone. However, it is well known that cryt.hrocyte production is depressed after the transfusion of large numbers of red blood cells, amid it seems probable that the erythrocyt.ic tissue will prove to be similar to the granulocytic tissue mi possessimig a chalomie mechanism (see Rcfs. 218, 295, 464). If this is so then the chalone may be produced in the circulating erythrocytes amid it may inifluemiceonly the mitotic activity of the crythrocyte progenitor cells. If such a mechanism is present in both the gramiulocytic and erythrocytic systems, it follows that these systems may show a reduced mitotic rate during stress, and pos sibly even a diurnal mitotic rhythm. Similarly, they may show changes in the mitotic rate after adrenalectomy or after injections of adrenalin or glucocorticoid hormones. It is unfortunate that the literature on these various points, while voluminous, mainly records variations in the num bers of gramiulocytes and erythrocyt.es in the blood, and these may commonly depend on processes other than the mitotic rate (see, for instance, Rcfs. 27, 56, 199). The granulocytic and erythrocytic tissues differ from most other tissues so far considered in that they must be able to adjust their rates of cell production not only to changes in tissue mass but also to changes in functional demand. In the case of the gramiulocytes, it is evident that mature circulating cells arc eliminated riot according to their age but according to the work they have to do in combatting bacterial invasion, since in these cells func tiomi leads to death (122, 422, 484). In the case of the erythrocytcs the mature circulating cells are eliminated, as in a normal tissue, according to their age, but the cell numbers must be able to imicrease if, for any reason, the oxygen supply to the tissues becomes inadequate (see Refs. 263, 464). In both these tissues it is evident that these adjustments are made through the actions of the 2 l)Oiet.inis. The sites of synthesis of these 2 subst.amiccs are not yet certain, but granulopoictin may be produced in functioning or postfunictioniing granulocytes,6 while crythropoietin may be produced in the kidney or other tissues (264, 351, 384, 411). Vol. 25, November 1965 quire different controlling mechanisms,― and it is worth considering what these might be. If the stem cells arc indeed pluripotent, this may imply that, unlike most tissue cells, they contain more than 1 set of genies that are potentially capable of directing the maturation of more than 1 type of cell. It appears in fact that in these cells the final step in differentiation has not been taken and that when it is taken, whether in the direction of the granulocyte or of the crythrocyte, the proc ess must be akin to tissue induction in the embryo. In their final form the cells may be expected to contain only 1 functional set of genes, which will lead to only 1 type of maturation. From what is now known of the chalones it seems most improbable that they could control this type of induction, and indeed specific chalonc synthesis may itself be the consequence of a specific type of induction. It is the poie tins that evidently act as inducers. Erythropoietin has no significant effect on either the mitotic activity or the rate of maturation of the erythrocytic progenitor cells (7, 294) ; its primary effect is merely to promote the con version of the stem cells into erythrocytic progenitor cells (7, 294). In a similar way granulopoietin appears to pro mote the conversion of stem cells into granulocytic progenitor cells. The probable relations of the poietins and chalones of the erythrocytic and granulocytic systems arc illustrated in Chart 9. Thus the actions of the poietins are fundamentally dif ferent from those of the mitogenic hormones, although both groups of substances are similar, first, in that they arc produced in response to a trigger mechanism operated by circumstances external to the animal, and second, in that they both induce the increased mass of their par ticular target tissues. This theory leaves unanswered the problem of mitotic control in the stem cells themselves, but this is a problem that can hardly be attacked until these cells have been recognized and localized. Possibly they may synthesize A second and more surprising way in which the granulo cytic amid crythrocytic tissues appear to differ from ordi nary tissues is that throughout life both may continue to be formed from a commomi stem cell l)oPulationi, which may j)erhaps be located in the bone marrow. It is even possible that the stem cells may form part of the lympho cytic system (see Ref. 533). Although in the adult mam mal the stem cells may prove to comprise 2 or more sepa rate groups, each leading to only 1 mature cell type, the Present evidence is generally held to indicate that they are pluripotent cells (29, 293). If this is accepted then, u.s Lajtha (292) has pointed out, there are 2 ways in which an increased number of mature cells may be ob taimied. First, an increased number of stem cells may differentiate to enter either the granulocytic or the cry throcytic tissue systems, and second, there may be, imicrease in the number of mitoscs through which each maturing cell passes. Lajtha concludes that “itis quite l)Ossible arid indeed likely that the two mechamiisms re E@D Gran.D CHART 9.—Diagram illustrating the probable method of con trol of cell recruitment in the erythrocytic and granulocytic tissues. The stem cells (8) differentiate into erythrocytic pro genitor cells (P) under the influence of erythropoietin (E.P.), and into granulocytic progenitor cells (P) under the influence of granulopoietin (G.P.). Mitotic activity is limited in the erythro cytic progenitor cells by chalone (E.Ch.) from the mature dying erythrocytes (Ery.D.), and in the granulocytie progenitGr cells by chalone (G.Ch.) from the mature dying granulocytes (Gran.D.). BuLL0uGH—Mitotic their own chalonc or possibly they may respond to one or all of the chalones of their daughter tissues. Jun this cominiectiomiit may be imj)Ortant that the mitotic activity of lymphocytes, which are evidently stem cells, can be suppressed by granulocytes (123, 124), possibly through the action of the granulocytic chalone. 4. Mitotic control in lymphoid tissue.—Froni a comi sideration of a wide range of evidence, Yoffey (533) has suggested that although the lymphoid tissue may appear to be entirely separate from the granulocytic and eryth rocytic tissues, in fact all 3 may together constitute 1 large tissue system—the lymphomyeloid complex. He has concluded “thatthe different parts of the complex are in close functional relationship, and that through the blood stream there is in all probability a constant miter change of cells between them.― At the moment this remaimis only an imiteresting possibility. The lymphoid system is obviously highly complex, but it evidently produces omily 2 types of “mature― cell, the small lymphocyte amid the plasma cell [see the review by Yoffey (534)]. The production pathway of the small lymphocyte is often believed to begin with the ret.icular cell, which remains static in the lymphopoictic center amid which, by a series of successive mitoses, gives rise to the large lymphocyte, the medium lymphocyte, and finally the small lymphocyte. If this is the basic mitotic system of the lymphoid tissue, and if the small lymphocyte is considered to be a “mature― cell, themi the system displays the usual pattern, whereby increasing maturity is matched by decreasing mitotic activity. However, it is by no means certaimi what role the small lymphocyte plays in the body. Its rate of j)roductiori is high. hi the thymus Leblond and Saimit.e-Marie (305) consider that the division of 1 rcticular cell usually results in the production of 128 small lymphocytes, and iii the mesemiteric lymph node Grundmann (214) estimates that 1 reticular cell may give rise to 64 small lymphocytes. This rate of production argues either a short life-span or a rapid transformation into some other type of cell, or both. All that is known is that large numbers of small lynipho cytes migrate via the lymph amid the blood to the tissues, and it is believed that large numbers may settle in the boric marrow (206, 207). This system is essentially similar to that seen in the erythrocytic and gramiulocytic tissues, and this suggests the possible existence of a chalone control mcchamiism. There is no direct evidence to support this suggestion, but considerable indirect evidence has been reviewed 1707 and Functional Homeostasis by Dougherty (139), who shows that the glucocorticoid hor mones, administered in excess, result mi an involution of the lymph nodes, thymus, and spleen that is characterized by a sharply reduced mitotic rate and a lower lymphocyte output. The same effect is seen after any kimid of stress, and there is also a marked diurnal rhythm in the lympho cyte content of the blood. These stress effects are elimi nated by adrenalectomy, which increases the mitotic rate and raises the number of lymphocytes in the blood while simultaneously reducing their life-span. Furthermore, during recovery after several hr of imposed stress, the blood lymphocyte content not only quickly returns to normal but for a few hr exceeds it, an effect which could be the re sult of a stress-induced accumulation of cells in aiit.cphase. It is evidemit that all these effects are closely simiiilar to those described for epidermis amid other tissues in which they are dependent on the existence of a cha@mie mech anism. The 2nd and apparently separate niitotic system of the lymphoid tissue is that by which the plasma (Yellis pro duced. Leblomid amid Saimite-Marie (305) regard this as arm alternative form of maturation to that which gives rise to the small lymphocyte, and they describe the sequence of niitoses and of cytoplasmic maturation which they be lieve plasma point to occur between the large cell (see also Ref. 369). of view expressed lymphocyte and 1mmsharp contrast by Yoffey the is the (533, 534), who regards the plasma (Yellas being derived from the small lym@)ho cyte (see also Refs. 205, 208). He describes the way in which this cell grows imi size amid becomes transformed into a pyroniiniophilic cell, which thermundergoes successive mitoses to form a group of daughter plasma cells. Cer tainly it appears that the small lymphocyte can be stiniu lated to grow in size and undergo mitosis both in vivo (205, 390) amid with @)hytoheniagglutimiini in vitro (338, 371). It is now generally agreed that the function of the plasmiia cell is the synthesis of amitibody. Whether the small lymphocyte or the ret.icular cell is the l)aremit (Yell,it. is the sudden stimulus of the invading antigen that results inn the production of the pyronimiophilic cell amidin the burst of mitot.ic activity that ceases only when the I)hLsma cell matures amid fills with antibody (22). It is well known that each invading antigen calls forth the production of an appropriate antibody, arid therefore it must be con eluded that each plasma cell is narrowly committed to only 1 program of protein synthesis. Yoffey (534) has de scribed the plasma cell as “conditioned―amid has com merited that “whatever theory of antibody production may ultimately prove to be correct, it. is clear that inn the primary response there is some conditiorunig of the lymnph oid cells, whether genetic or otherwise.― Medawar (343), expressing a similar l)Oimitof view, has emphasized that “theantigen, like the inducer, is armagent that commits a cell to a certain pathway of differentiation—to one path way among several that it might have taken, arid that lie within the gemictic capability of the cell.― This point. of view is closely similar to that expressed above when the actions of the l)Oietimiswere likened to those of embryonic inducers. If it is temit.atively accepted that the small lymphocyte may be the immuniologically competent cell therm a specu lative theory can be developed as follows. The supply of smnall lymphocytes is maintained by the mnitotic activity of a population of stem cells, which are the larger lympho cytes amidthe reticular cells. To maimitaimiitself mi balance this population must be controlled by sonic feedback mcchamiism that limits the mit.otic rate amid promotes the maturation of the small lymphocytes. The role l)layed by the adrenals indicates that this may prove to be a typical chalone mechanism, arid if so then the system will be self-balancing simice the loss of small lymphocytes, whether by death or differentiation, will lead to a lowered chalomic concentration in the blood arid lymph. 1708 Cancer Research On this view the small lymphocyte is regarded as a cell in which the final step of differentiation has riot been taken and which therefore still possesses a number, perhaps a very large miumber, of gemictic possibilities. The anit.igcmi, acting as arminducer, limits these possibilities to only 1—the synthesis of the appropriate amit.ibody—and at the same time evidently frees the cell from the mitotic control that had previously held it inert. If final differ enitiation always includes an acquired ability to synthesize a n@pecificchalomie, then a newly differentiated cell may always lack sufficient chalone amid therefore always under go mitosis until, in the plasma cell, the intracellular chalomie concentration rises to ann effective level. Once again, although there is no direct evidence available to support the theory that a chalonie mechanism is present, there is some indirect evidence derived from a study of the actions of glucocorticoid hormones. White (522) has recently reviewed evidence that these hormones reduce the production rate of the plasma cells, but not the rate of antibody production in army cells already formed. A plasma cell is immunologically active arid, being fully differentiated, it is unable ever again to revert to a state of immunologic competence. Imideed it is generally believed that plasma cells may be short lived (121, 369, 437). The basic response of the lymphoid system to anit.igen stimulation is in fact the creation of a new type of tissue that was riot present in the body before, and although the plasma cells may all belong to type D (see Chart . 4) amid therefore die, some cells, perhaps of type 1%!,may survive for long l)criOds. These lomig-lived cells may superficially resemble the short-lived umiconditioned lymphocytes (see Refs. 168, 207), amid they may be able to take l)art in a secondary response. Such a respomise would then involve a double reaction : the increased ac tivity, perhaps including mitosis as well as amitibody pro duct.ioni, in already existing tissue cells; and the induction of new cells into the tissue. This would imply that an antigen may normally act, directly or indirectly, on both the induction and the production of the mature plasma cells. These suggestions tend to run counter to the clonal theory of Burnet (95, 96), at least when this theory is stated innits most extreme form. Indeed, an outstanding unanswered question of the momenit is the actual degree of competence of the immunologically competent cells. As mentioned above, it. has even been suggested (see Ref. 534) that such cells may be imiduced by the poietins to give rise to either granulocytes or erythrocytes. The bone marrow contains large numbers of small lymphocytes, and there is evidemice to suggest that they may be induced to differentiate in this way. The most sigmiificant argument against this suggestion arises from the evident inability of t.horacic duct lymphocytes to repopulate bone marrow from which the cells have been eliminated by irradiation (see Ref. 186). Although much of this discussion of the mitotic activity of the lymphoid tissue is speculative, one important point is clear. As was the case with the granulocytic arid eryth rocytic tissues, the lymphoid tissue is capable of adjusting its mitotic rate both to the demands of tissue mass, which may be mediated through a chalone mechanism, and to Vol. 25, November 1965 the demands of function, which are mediated through the inducer-like action of an antigen. With the great variety of possible antigens, such a system must be unusually corn plex, amid the homeostatic mechanisms controlling mitosis amid cell maturation are also likely to be more complex than in any other tissue system of the body. 5. The growth of hair.—It has been suggested above that the relatiomiship of tissue mass to body mass is a function of the rate of chalone production in the mature cells. The question now arises how the mitotic rate may be controlled in such a tissue as hair, in which the mature cells die so rapidly that no significant mass of chalone producing cells can be built up. The high mitotic ac tivity of the hair bulb results in the production of a shaft of dead cells, which are obviously incapable of chalone production, but when the hair reaches its correct length, or mass, the niitotic activity ceases. It is obvious that some mechamiism must control the phases of activity and imiactivit.y of the cells, and since it seems unreasonable to expect that. this mechanism may be unique, it is worth considering how a typical chalone mechanism might be modified to produce this effect. The complex l)attern of hair growth has often been de scribed (see Refs. 80, 112, 357). Reduced to its simplest terms it involves 4 successive phases of inactivity and activity. In the 1st phase the cells of the hair bulbs are in a resting comiditioni (called telogen) mi which no mitosis is ever seen ; in the 2nd phase (early anagen) the cells show inicreasimig mitotic activity leading to the lengthening of the follicle ; in the 3rd l)hase (anagen) the cells show con tinuous high mitotic activity, and it is the duration of this phase that determines the length of the hair; in the 4th phase (catagen) the mitotic activity decreases and the follicle shortens to enter the resting condition once more. In any 1 area of skin all the follicles may follow this rhythm in unison, as is the case in mice and rats (141, 142) ; alternatively, each follicle may follow its own in dividual rhythm, as is the case in guinea pigs and men (1 12) . In either case it seems that the control mechanism must be intrinsic in the individual follicle and not systemic (141,142). Considering first the resting follicle, it is evident that the cells are similar to those of any ordinary inert tissue in that they are able to react by high mitotic activity to any form of wounding, and thus it appears that they must normally be controlled by a high chalone concentration. In contrast, the cells of the actively growing follicle show an extremely high rate of mitosis, a mitotic cycle length of only about 12 hr (80, 111), and a complete lack of re sponse to stress or adrenalin.2 Indeed the cells react as though little or no chalone exist within them. One obvious possible explanation for the rhythm of hair growth lies in the suggestion that it depends inversely on a rhythm of chalomie production, and this agrees with the suggestion of Chase (112) and Chase and Eaton (113) that the onset of hair growth may be due to “amore or less rapid loss or leaching of an inhibitor.― This theory is expressed diagramatically in Chart 10, and the evidence both for and against it has recently been summarized by Ebling and Johnson (149). If this theory is basically correct then it should follow BuLL0uGH—Mitotic teloqen anaqen C C.) C 0 C., @ and Functional Homeostasis teloqen I a) C 0 0 C., trol CHART 10.—Diagram of the hair growth illustrating the possible cycle by the fluctuating manner chalone of con concen tration in the hair root cells. that the adrenal hormones should be without army effect in either the resting or the actively growing phases, but. should exert a pronounced action during the 2 transition periods. Thus, for instance, when the chalone concemitra tion falls towards that critical level at which mitot.ic ac tivity begins, the enhancement of the efficiency of the chalone complex by stress or by adrenalin injectiomi should delay the onset of the phase of hair growth, while the weakening of the efficiency of the chalone complex by adrenalectomy should accelerate it. As regards stress, Blumenthal (47) has shown that when mice arc main tamed on a reduced diet the initiation of new waves of hair growth is delayed or even prevented (see also Chase, 112). As regards the adrenal hormones, Mohn (352) amid Ebling and Johnson (149) have shown that the initiation of hair growth is accelerated by either hypophysectomy or adrenalectomy and that it is delayed by injections of ACTH, cortisone, or adrenalin. Once again it appears that a glucocorticoid hormone may be involved as a 3rd partner in the control niechamiism. Thus the available evidence tends to support the theory that hair growth may be controlled by the normal type of mechanism, but, whereas in other tissues the rate of chalone production may be relatively constant, in the hair bulb it appears to be rhythmic. This rhythm may be inherent in the genes directing chalone synthesis or it may be imposed or modified by factors in the follicular environment that affect the activity of these genes. The available evidence strongly suggests that the ultimate control lies in a gene or group of genes that oscillate be tween activity and inactivity. For any particular type of hair the durations of the various phases of the rhythm arc evidently constant, but between different types of hair they vary widely. Oscillating genetic comitrol of this type may be analogous to the oscillating genetic control of estrous cycles (see Ref. 215). It is, however, evident from the latest results of Ebling and Johnson (148, 149) that the follicular environment may exercise a moderating influence on the basic hair growth rhythm. In a similar manner the follicular en vironment may modify gene expression at the agouti locus within the melanocytes associated with the hair root (43, 446) ; perhaps the most spectacular example of this is in such northern species as the varying hare and the arctic fox. In these the color of the growing hair is dependent on the expression of the melanocyte genes, which is de termined according to the season by the physiologic condi tion of the animal. 6. Conclusions.—Fromthis survey it appears that the 1709 mechanism of mitotic and functional honmeostasis in certain adult mammalian tissues is subject to a 2mid order of con trol by mitogenic hormones amid that in a few tissues the situation is also modified by the actions of l)Oietins or antigens that augment the tissue mass by imiducinig the final differentiation of stem cells. Regarding the mitogenic horniones, it. is clear that. be cause of the effective inverse balance between cell gain and cell loss a great increase in the mitotic rate is needed before army significant chamige in tissue mass cant occur. It seems that the effects of these hormones are dramatic maimily because the target tissues inn their unst.inmulat.ed comiditiomi connmomily show rio niitosis at all. Their manmier of action supports the suggestion that they niay mieutralize the chalonies of the target tissues, arid their actiomi on the genonie is therefore indirect. It is miter esting that in insects Kroeger (288) has also concluded “thathorniomies . . . camimiotact directly on the genetic loci, arid that there must be an intermediate system which relates the hormonal stimulus to the lOci.― It seems possible that the hormone-dependent. t.is@ues are merely those that are characterized by armexaggerated dependence on hormones, which also oj)crate to sonic degree in all nornial tissues (63) . The evidence suggests that target-tissue specificity is expressed in a quantitative rather than a qualitative manner, and regarding the sex horniones Bullough (63) has emphasized that. most tissues may jossess some sensitivity, which gives them the po temitiality for enlargement inn relation to the sexual proc esses should the riced ever arise. Examples of accessory sexual tissues that seem to have evolved inn this way are the posterior region of the stickleback kidney, the thumnb pads of the frog, the comb amid wattles of the cock, the sexual skin of the monkey, amid the mammary gland. In all such cases it is evidently the tissues that become modi fled, through changes in their nmitot.ic amid functional homeostatic mechanisms, to emiable them to react more strongly with the horniones. The hormones themselves have evidently remained unchanged. It must also be emphasized that the mitogenic hormones probably exert a wide variety of actions inside cells, amid that only one or sonic of these are directly related to mitosis. It is interesting that all the known mitogenic hormones, such as androgemis, estrogens, prolactin, amid thyrotropin, are under the control of the anterior pituitary gland, which is itself imifluemicedby the nervous system amid thus by inforniation entering through the sense organs. Al though mmmost laboratory mammals the rate of secretion of the mitogenic hormones may be wholly controlled by inherent genetic mechanisms, as mi the case of the estrous cycle (see Ref. 215), in most wild mammals the rate of secretion of these hormones is modified by environmemital changes that act as trigger mechammisms. This is part.icu larly obvious in relation to seasonal breeding (@yclcs (see Ref.72). It is interesting to note that in laboratory animals the mechanism comitrolling hair growth is also evidently genetically comitrolled, while in most wild mammals the growth of a new coat is a sea.sonmal phenomenomi. Here again gene action is evidently modified by seasonal changes. However, although the time of onset of hair Cancer Research 1710 growth may be determined in this way, the emidof anagen appears to be determined solely by gene action. It, has been stressed that increased tissue mass cannot easily be obtained through imicreased mitotic activity but that in the granulocytic and erythrocytic systems in creased tissue mass is sim@)ly achieved by the incorpora tion of new cells that were not previously part of these tissues. This is essemitially an exploitation of an em bryonic mechanism whereby, under the direction of either graniulopoictin or erythropoietimi , relatively unidiffer enitiated stem cells are induced to undergo their final dif ferenit.iatiomi. The i)Oietimis, like the antigens that act in a similar manner on the relatively undifferentiated, im munologically competent lymphocytes, are also part of a niechaniismii whereby the animal can respond to emiviron mental change. Although the manner of control of eryt.hropoietin production is not yet clear (see Refs. 20, 379), granulopoietimi production seems to depend on the rate of granulocyte destruction, which is a function of the work the graniulocytes have to do. Similarly in the case of the lymphocytes, antigen induction is de l)emident oh unpredictable circumstance. It is evident that mammals possess only limited stem cell populations, amid iii this they differ markedly from many amphibianis in which the regeneration, for in stance, of entire new limbs indicates the existence of stem cells with great potentialities. However, the recurrent growth of antlers in deer has not yet been analyzed from this l)Oinmtof view (see Refs. 201, 202). C. THE BREAKDOWN OF THE i\IECHANISM 1. The mechanism itself.—The essential features of the theory of mitotic amid functional homeosta.sis outlined above are summarized diagrammatically fri Chart 11; for simplicity, regulator genes are not included. The process of induction, by which the tissue originated, in volved the suppression of all genes that are relevant to other types of tissue (the “blockedgenes―). The remain inig genies that are, or may become, functional form the CHART 11.—Diagram illustrating Vol. 25, November 1965 facultative genome (170), and they include genes that dictate the synthesis of general metabolic enzymes (the “essential genes―), genes that dictate the synthesis of those special enzymes on which the tissue organization depends (the “tissuegenes―), and genes that dictate the synthesis of those enzymes that underlie the mitotic cycle (the “mitosis genes―).In the chart the syntheses con trolled by the tissue genies and the mitosis genes are re garded as occurring in a series of steps, each of which is under separate gene control. In each case only one synthetic pathway is indicated although, of course, there must be many. One group of tissue genes (the “chalone genes―) are regarded as specifying both the structural details amid the rate of synthesis of the tissue-specific chalone, which then promotes the activity of the remain inig tissue genies. If the chalone concentration falls (as in wounds) not only does this promotion cease but many of the already functional cells lose some or all of their special characteristics. It is not clear whether in pro moting tissue specialization the chalonc acts alone or whether adrenalin (and perhaps a glucocorticoid hormone) is also involved. It is, however, clear that the power of a chalone to inhibit the activity of the mitosis genes is strengthened by adrenalin. Since it seems reasonable to believe that the mitosis genies may be the same in the cells of all tissues, it is difficult to understand how within any one tissue they could develop the ability to respond only to the chalone of that tissue. One possibility is that a tissue-specific, chalone-sensitive trigger mechanism may operate at the beginning of the l)haSe of DNA synthesis and another at the beginning of the phase of mitosis. Both these phases, once begun, are self-supporting. Finally, it should be emphasized that although a chalone-adrenalimi complex appears to be a central feature of the homeostatic mechanism, the effects of withdrawing chalone and those of withdrawing adrenalin are very different. When most of the adrenalin is withdrawn (as after adrenalectomy) the epidermal mitotic rate rises 2- the homeostasis in a typical mammalian tissue. theory of mitotic and functional For explanation see the text. BuLL0uGH—Mitotic and Functional Homeostasis or 3-fold (83) ; when most of the chalone is withdrawn (as after wounding) the epidermal mitotic rate rises perhaps 10- to 50-fold (81). Also, the adrenalin effect is im mediate, evidently because this hormone is so rapidly gained or lost, whereas the chalone effect takes many hr, perhaps because it is more stable and less easily lost, but perhaps also because it may act most powerfully in early prosphase. Although in Chart 11 the situation shown is obviously oversimplified and inadequate, it is at least useful in in dicating some of the main points at which the homeostatic mechanism may be broken, either temporarily or per mamiently. 1711 normality is usually associated with chronic niyeloid leukemia (58). If the gene damage leads to the production of “foreign―proteins, then presumably the immune mechanism will be activated, arid as Burniet (95) has sug gested, this may be one of the main defences against cancer. If, however, the mutation leads to the weakening and ultimate cessation of genie action therm the immune niechaniisni will presumably not operate, arid there is indeed considerable evidence that tumor cells are deficient in specific proteins amid show a loss of immunologic spe cificity (see Ref. 93). In a carefully reasoned criticism of the theory of somatic mutation, Rous (415) has emphasized the relative lack of 2. Carcinogenesis.—The effects of a temporary break evidence that such mutation does commonly occur arid down of the homeostatic mechamiism have already been has also stressed the important. loinit that all carcinogens considered at lemigth ; the question of a permanent break arc riot miiutagenis arid all mutagenis are riot carcinogens. down leads to a consideration of the relation of the chalone This latter point is also emphasized by Trainini et al. (489), theory to the cancer problem. The current theories of who however conclude that it is “probable that only a carcinogenesis have been reviewed and criticized by small proportion of mutagenmic agents would share the Hiegcr (241, 242), but even more valuable for present (@omnioni @@ite or sites of action which one would postulate purposes arc the recent statements by Foulds (170—72). to be involved in the initiation of carciniogenesis.― It is clear that one of the most important theories con Hieger (241, 242), too, has objected that genie daniage cerning carcinogenesis derives from the work of Bereni must. be a comparatively rare-event and that. if, for cancer blum (34) amid of Rous amid Kidd (416), who distinguished to develop, several distinct niutationis are needed, u.s between a process of initiation, whereby a number of indeed seems to be the case, therm the chances of inntiation dormant amidunrecognizable tumor cells are formed within decrease “t.opractically zero.― However, Burniet (94, the tissue, and a process of promotion, whereby these 95), while agreeing that “at.any given genetic locus an dormant cells are caused to multiply to produce a visible error mi replication occurs with a frequency iii the range tumor. Initiation, which is irreversible, may perhaps 10@—10@per replication,― has pointed out that. in ann occur spontaneously or may be the result of any kind of animal as large as a man some 10'@cells miiay be constantly carcinogenic action ; l)rOniOtiOn, which at least in its early reproducing themselves. However, all such arguments stages is reversible, may also be spomitaneous or may be omit a number of innportanit considerations. First, they hastened by a promoting agent. The inadequacy of such do riot take into account the effects of carcinogenic agents a simple analysis has been stressed by Foulds (171, 172), in increasing the mutation rate (see Ref. 296), arid it must who has pointed out, first, that the action of a carcinogen be emphasized that many so-called spontaneous tumors is quantitative. With smaller doses fewer tumor cells may have been induced in this way by as yet undetected are produced, and these, after promotion, develop only carcinogens. Second, they do not consider the POSsibilitY into papillomas, nearly all of which regress. With larger that genie damage, whether spontaneous or induced, may doses more tumor cells are iroduced, amid these, after occur in nondividing cells, amid indeed Curtis (127) has promotion, give rise either to papillomas with the lower presented evidence that nondividing mammalian cells to progress into carcinomas or occasionally to carcinomas accumulate deleterious mutatiomis at such a rate that by ab initio. Second, Foulds emphasizes that, whether spomi late middle age “virtually all cells carry many genie niuta taneously or after treatment with carcinogens, tumors may tions.― Third, they omit consideration of the Possibility that gene damage or failure may be at least partly de arise at one or many sites within a tissue, appearing at pendenit on inherited genie weaknesses, amid that mmsuch random both in space and time, and that occasionally they animals as man it may even be “thatthe majority of may even involve a whole organ. Third, Foulds con malignancies are of this kind― (see Refs. 91—93). eludes that a benign tumor is subject to 4 possible fates If for any of these reasons gene function is impaired “namelyregression, indolent persistence, growth without then the reaction of the damaged cells would be expected qualitative change, and progression to malignant neo to vary according to the type of genie or genes involved. plasia―,—but that the commomi tendency is for any tumor For the present argument the simplest situation would be to become progressively more malignant. one involving some failure mi the genes that determine To explain the cellular chamiges that underlie these the production rate or the structure of the tissue chalone, phenomena one widely held theory is that initiation in and even a relatively slight failure would tend to give the volves 1 or more somatic mutations, and it is evident that cells some proliferative advantage. According to the these mutations might involve damage to the genes that Darwinian point of view advocated by Burnet (96), this previously controlled mitotic and functional homeostasis (55). In Haddow's (220) words, it is possible to “regard might seem sufficient to ensure the appearance of a tumor, but in fact it is now clear that certain couniterchecks would the problem as one of the impact, upon the genomal in be expected to come into operation to limit amid even to tegrity, of any of a host of reagents―, and it is now evident First, if only single that similar damage can be inflicted on cells in vitro (25, prevent any extra mitotic activity. cells, or small groups of cells, are damaged, the chalone 26). It is even known that a specific chromosome ab 1712 Cancer Research concentration within them should remain miormal because of diffusion inwards from the surrounding undamaged cells. An increased mitotic rate should not be seen until the group has grown to such a size that its central cells are beyond the effective range of chalone diffusion, amid judging from the length of the chalone diffusion path ad jacemit to a wound (81), this critical cell mass might have a diameter of a little less than 1 mm. Beyond this point, so long as the rise in the mitotic rate is only moderate, a 2nmd countercheck might begin to oj)erate. This would depend on the expectation that any rise in the mitotic rate would be offset by a reduction in the life expectancy of the mature cells. Thus the latent period described by Bereniblum (34) and Rous amid Kidd (416) may include the whole of the period from initiation to the end of the period of effectiveness of this 2nd counter check. This question is considered further in the next sectiomi. So far only the expected consequences of damage to the chalonie genes have been considered. It. is obviously possible that genie damage might also involve, or even only involve, those other tissue genies that dictate the synthesis of the typical tissue proteins. The result of this would be expected to vary widely according to which genes were damaged amid in what degree. In the 1st place the damage might involve the genie operators through which the chalommemechanism ultimately acts, and this could have similar consequences to chalone lack. Second, the damage might upset the balance between cell gain and cell loss either by reducing the rate at which the tissue genies synthesize tissue protein (183, 414) or by increasing the life expectancy of the mature cells. It seems probable that delayed or inadequately realized maturity may in crease the proportion of the cell population that remains capable of division, amid in this connection Berenblum (36) has emphasized that promoting agents commonly appear to operate “byproducing a delay in maturation of the dormant tumour cells―. Regarding the life expectancy of the mature cells, it is known that this is enhanced by the chalonme complex, but it is possible that this action may operate through a mechanism that might be sepa rately subject to damage. In the 3rd place, the variety of possible damage to the tissue genies could account for Foulds' (170) conclusion that “tumoursderived from the same tissue by the same carcinogenic procedure may be extremely varied and intergraded― so that “itis probable indeed that no two tumours are exactly alike in every respect.― Foulds (170) also emphasizes that tumor characters are not static but tend to change progressively, that such progression occurs independently in different characters in the same tumor, that it occurs in tumors that are miolonger growing, and that it may be continuous in large numbers of small steps or discontinuous in small numbers of large steps. Commonly such progressive changes evidently also involve further gradual breakdown in the mitotic and functional homeostatic mechanism, which may lead to greater malig nancy. Although the evidence is still inadequate, the view of Klein and Klein (279, 280) that this is due to progressive gene damage may be tentatively accepted. One effect of such tissue gene damage might be seen at Vol. 25, November 1965 the cell surface. It is commonly believed that the cell surface structure is tissue specific, with the result that the cells of each tissue tend to hold together. With pro gressive malfunction of the genes controlling surface structure, a point may be reached when that structure becomes so modified that the cells separate and metastasis results. One final possibility may be mentioned—namely, that the genie damage might involve the mitosis genies. In this case it is probable that the cells would be unable to divide or that they would die during attempted division, and it is indeed one of the aims of radiotherapy to inhibit tumor growth in this way. From this very brief review of the evidemice it appears that both initiation and progression may involve sudden amid irreversible changes in the genome ; that with increas ing age such gene damage seems to occur at random in most if not all tissues; that this process may be augmented by carcinogens and supported by imiherited gene weak niesses ; and that if, by any route, such damage leads to malfunction of the mitotic amid fumictional homeostatic mechanism, so that the balance between cell gain and cell loss is disturbed, a tumor may result. 3. The chalone complex and the latent period.—Between the process of initiation amid the appearance of a tumor there is the latent period, which can be shortened by the application of a promoting agenit.. This process of promo tion is usually neither sudden nor irreversible, and it is importarTit to question whether the speed at which it pro ceeds may be related to the state of the mitotic homeo static mechanism, not only in the damaged cells but also in the surrounding normal tissue. It has already been noted that the latent period may be shortened either by hyperplasia (220, 242) or by a delay in the maturation of the dormant tumor cells (36), and it has also been em phasized that in normal tissues any weakness of the chalone complex leads both to increased mitotic activity and to delayed maturity. The length of the latent period is evidently determined by chance, and in most epithelia there is the added possi bilit.y that the damaged cells may be shed. Since, if the ammimallives long enough, all those latent tumor cells that are not shed may produce tumors, the difference between an increased chance and a decreased chance should lie mainly in an earlier or a later average time of appearance of the tumors. The question to be answered, therefore, is whether with an inefficient chalone complex, as after tissue damage or adrenalectomy, the latent period will be shortened, while with an efficient chalone complex, as during stress, the latent period will be lengthened. 4. Promoting agents.—Totake first the question of tissue damage, there is an old hypothesis that, given suffi cient time, irritation or ulceration or scarring automati cally leads through hyperplasia to cancer. Although this theory of the origin of cancer is inadequate today (242), it does remain possible that an increased mitotic rate may increase the chance of a genetic mistake and tend through chalone lack to favor the multiplication of any tumor cells that may be so formed. There is certainly strong cvi dence that epidermal woumiding or irritation will promote the earlier appearance of epidermal tumors in carcinogen BuLL0uGH—Mitotic and Functional Homeostasis treated skin (175, 176, 327, 397, 398, 416, 417, 450). Similarly it has been reported that regeneration following partial hepatectomy promotes the earlier appearance of tumors in carcinogen-treated liver (194, 303, 304) and that primary hepatic carcinoma occurs with greater frequency in cirrhotic livers. However, both wound repair amid re generation are normally short-lived episodes, amid it is therefore not surprising that a stronger promoting action can be obtained by repeated tissue damage (397, 398). A 2nd way in which mitotic homeostatic mechanisms may be damaged is by adrenalectomy, and because of the increased mitotic rate this may also be expected to act in the manner of a promoting agent. Unfortunately most investigations have failed to distinguish between the proc esses of initiation and promotiomi, or have related solely to the growth of established tumors, which may be adversely affected by the general metabolic disturbance (32, 33, 409, 469). Recently, however, Trainin (488) has made a careful analysis of the effects of adrenalectomy amid has shown that, although it has no effect on initiation, it strongly enhances promotion. This is in agreement with Law (302), who found that adrenalectomy increases the incidence of spontaneous lymphoid leukemia, amid with Metcalf (349), who found that high leukemia AKR mice are characterized by low productiomi of glucocorticoid hormone. The 3rd type of promoting agemit includes irritant chemi cals, such as croton oil, which are often considered to act through cell damage and consequent hyperplasia. How ever, the theory that hyperplasia is the essence of promo tion has been critically discussed by Bercnblum (35, 36), who has suggested that promotion must depend on a dislocation of the normal balance between cell gain and cell loss and that a promoting agent may exert its action more by preventing cell maturation than by promoting mitosis. On present evidence it is difficult to distinguish the relative importance of these 2 processes, amid it is possible that in different circumstamiccs either or both may have the same consequence, which is the build-up of a group of damaged cells sufficiently large for the central ‘u'eato be freed from chalone control. After successful promotion the tumor cells are com monly able to continue their multiplication without further assistance. It follows that the act of promotion must either have altered the nature of the tumor cells mm the direction of autonomy or have enabled them to realize characteristics they already possessed. As they emerge from their dormancy the tumor cells may give the impres sion either that they are lacking sufficient chalone or that they are no longer able to respond to whatever chalone is present. If the 2nd alternative is correct then the cells should be unable to respond to induced changes in either the chalone content or the adrenalin content of the tissue. In fact, however, it is evident that newly formed tumors are commonly able to respond to such changes. Thus Trotter (491) has shown that in the liver partial hepatec tomy results in a greatly increased mitotic rate in small adenomatous nodules (see also Refs. 185, 320), and further she has pointed out that as malignancy increases this power of reaction is lost. In a similar manner certain types of tumors continue to show a diurnal mitotic 1713 rhythm (317—19,505), while others that are perhaps more malignant show no diurnal rhythm at all (135, 197). It may therefore be concluded that both the dormant tumor cells and the cells of benign tumors may miormally be capable of responding to the chalonie mechanism. Indeed, evcmi after a tumor has appeared, it may still possess such a relationiship between cell gain amid cell loss that it may reach a new Poimit of balance, arid, at least for a time, it may remain mi the state of “indolent persistence― de scribed by Foulds (171). Commonly, however, a pro gressive loss of functional maturity may lead to a progres sive reduction in the chalone concemitration amid so to an increased mitotic rate, which is dependent both on a progressive shortening of the cell cycle length (410) amid a progressive increase in the proportion of cells involved in mitosis. In addition, those cells which do manage to ma ture should die more quickly, amid at the theoretical cx treme, growth would be explosive. An approximation to this extreme may be seen in many ascitcs tunmors, in which the cell cycle may be reduced to as little as 11.5 hr (132) and in which rio mature cells arc present. Un fortunately, relatively little precise imiformation is avail able on the mitotic rates or on the durations of the cell cycles of tumor cells as compared with those of their tissues of origin (see Refs. 132, 344). It seems probable that at some point during progres sion the cells may lose their ability to react to army re mainiing chalonic amid that from therm onwards they are fully autonomous. It has been found, for imistance, that malignant leukemic granulocytes may continue to produce a chalone-like substance capable of inhibiting mitosis in normal granulocytic progemmitor cells8 (423) but that they themselves are unable to respond to it (123, 124). With respect to those few tumors that are already malignant when they first appear, it may be assumed that their loss of ability to responid to the normal homeostatic mechanism occurred in 1 large step during either initiation or dor mancy. In either case promotion would be unnecessary and growth would be rapid. 5. Retarding agents.—If a weakened mitotic arid func tional homeostatic mechamiism is armessential feature of promotion then a strengthened mechanism may prolong the period of dormancy of the tumor cells. It is apparent that in those tissues, such as liver amid kidney, which have a naturally low mitotic rate, amid presumably therefore a naturally powerful mitotic control mechanism, the muta tion rate may be high (127) but the chances of promotion are so low that the cancer incidence is also low ; innthose tissues, such as epidermis, which have a weaker mitotic control mechanism, the chances of promotiomn arc much greater; while in those tissues, such as the basal cells of the duodenal crypts, which apparently possess a very weak mitotic control mechanism, the high chances of promotion may be powerfully offset by the high rate of cell loss. Curtis (127) has noted that, while a mitotically inert tissue spontaneous mutations build up to a high level, iii a mitotically active tissue they remain rare. The simplest way to strengthen the homeostatic mitotic mechanisms of the tissues is by means of stress, which leads to a higher rate of secretion of adrenalin amid gluco corticoid hormones. If the chalone niechamiism in the 1714 Cancer Research damaged cells is even partly active, this should retard the multiplication of the latent tumor cells and delay the growth of bemmigmi tumors. Direct studies of the effects of stress are few, but it has already been shown that a longer latent period is found in mice stressed by high tempera tures (179, 506, 507), low temperatures (513), or excessive noise (354), amid recently Anderson (14) has concluded that “apprehension [gives@ some protection against car cinogenesis.― Stressful conditions have also been shown to delay the growth of existing tumors, which may there fore have been relatively benign (289, 332, 407, 419). However, the most extensive amid important work on the effects of stress on carcinogenesis is that of Tannen baum and Silverstomie (475—78)and of Rusch et al. (418, 420), although none of this work was originally considered mitermsofstress. Tanmnmenbaum wasthefirsttoshowthat with a wide variety of latent tumor cells, both spontaneous arid induced, promotion is delayed amid even prevented by a restricted diet, but determined attempts to explain this effect in terms of the diet itself have failed. It has been noted, however, that such treatment results in an increase in the size of the adrenal gland (49, 432, 478) and in an inicrea.se in the secretion rate of both adrenalin (196) and the glucocorticoid hormones (182). A survey of the large literature on the effects of re stricted diets amid carcinogemiesis has been made by White (521). From this it is clear that riot only may promotion be prevented but the growth of what arc l)rObably rela tively benign tumors may be delayed (see also Ref. 248). One important observation, made by Pearson (382), shows that when undcrfecding retards the growth of an im planted tumor, the cells may show signs of reacquiring some degree of functional maturity, and this is, of course, exactly what would be expected with a strengthened chalonme complex if the process of progression towards malignancy had not gone too far. It is also clear that when progression has gone too far a restricted diet has no effect (521). If this retardation is indeed due to a strengthened chalone complex then a similar action should be exercised by adremialin or the glucocorticoid hormones. The effect of adrenalin does riot seem to have been tested, but the effect of the glucocorticoid hormones is well known. In particular, the careful work of Trainin (488) has clearly shown that, while hydrocortisone has no effect on initia tion, it strikingly imihibits promotion. The cortisone in hibition of spontaneous leukemia (267, 499, 529), of in duced skin tumors (28, 152, 187), and of induced skin and lung tumors (188) is probably due in each case to a similar inhibition of the process of promotion. Contrary cvi dence that cortisone acts to increase the yield of induced skin tumors (456) may be due to changed hair follicle activity (444). The effect of glucocorticoid hormones on the growth of existing tumors differs in different cases, but a reduced growth rate has been described in a lymphosarcoma (234, 463), in lymphoid leukemia (181, 244, 274), amid in various other tumors (465). However, such glucocorticoid-sensi tive tumors commonly progress to a state of glucocorticoid resistance (18, 297, 298). This change has the stable character of a mutation, amid it presumably denotes pro Vol. 25, November 1965 gression towards greater malignancy. There are several known cortisone-resistant types of tumor, but it is in teresting that their cells may still bind glucocorticoid hormones in a normal manner (98) . This may possibly suggest that the chalonc mechanism remains and that the cells have lost their power to react to it. Such evidence tends to confirm the conclusion that both latent tumor cells and relatively benign tumors may nor mally be subject in some degree to the mitotic homeostatic mechanism of their tissue of origin amid consequently that anything that strengthens this mechanism may delay the appearance and retard the early growth of a tumor. The only known retarding agemnts are glucocorticoid hormones, and it is obviously important to discover whether adrenalin and the various chalones are similarly active. Regarding chalones it has been noted that when excess blood, pre sumably containing the granulocytic chalone, is intro duced by transfusion, chronic mycloid leukemia may be suppressed (520) while acute leukemia is unaffected (123). Such unresponsive malignant cells may fail either to re spond to a normal chalone concentration or to produce significant amounts of chalone: the 1st alternative may be illustrated by malignant lcukemic granulocytes that continue to exert a chalone-like inhibition of mitosis in granulocyte progcmiitor cells,6 and the 2nd by other malig nant leukemic granulocytes, which are no longer able to inhibit lymphocyte mitosis (see Rcfs. 122—24). 6. Conclusions.—Thc theory that the processes of mi tiation and progression may commonly be due to gene damage, acquired or inherited, leads to the pessimistic view of the cancer problem described, for instance, by Rous (415) and Burch (93). In Burnet's words (94), “Everything suggests that, somatic cells being what they are, the impact of the environment must inexorably lead to an accumulation of mutant cells, some of which will have malignant descendants if life persists long enough.― However, it is already clear that the average time of tumor appearance may be considerably delayed by retard ing agents. The agents so far known are those adrenal hormones that are produced in response to stress and that act to strengthen the mitotic homeostatic mechanism. Their potential power has been strikingly illustrated in the experiments of Tannenbaum (473, 474), in which the latent tumor cells have remained suppressed throughout the entire lifetime of the animal. These experiments, which hold out the hope of large-scale cancer prevention, may yet prove to be some of the most important in the history of cancer research. It is also evident that re tarding agents that act by strengthening the homeostatic mechanism may delay the growth of visible tumors, at least as long as these remain in the earlier stages of pro gression. In the converse situation, the promoting agents, which advance the time of tumor appearance, may all act by weakening the homeostatic mechanism with effects that are evident in terms of mitotic rate, cell maturation, or cell life expectancy. One natural factor that may act in this way is increasing age. It has been noted in a number of species, including man, that from middle age onwards there is commonly a progressive degeneration of the adrenal cortex (130, 140, 268—70), and it has also been BuLL0uGH—Mitotic and Functional Homeostasis reported in both mice and men that middle age may be a time of increased mitotic activity (67, 487). However, the effect of middle age on adrenal function @i@not yet elear, and the situation is further confused by the differing reactions of individuals and of strains within 1 species (486). Little is known of changes in the rate of adrenalin secretion, although in men Kärki (275) has comicluded that “theadrenal medulla maintains its activity @pto a high age,―while in mice Bullough arid Laurence2 have indirect evidence indicating that the adrenalin output may be much reduced. In the case of the glucocorticoid hor mones the evidence is also inconclusive. On the one hand, it is generally agreed that mmman the rate of cx cretion of the hormone derivatives declines progressively during middle age (224, 278, 519), but on the other, it appears clear that this does not reflect any significant. change in the plasma hormone level (246, 519). It up pears that a reduced adrenal secretion rate may be offset by an impaired capacity to metabolize arid excrete the glucocorticoid hormones and possibly also to utilize them. Although this is a matter that needs further investigation, it remaimis possible that in middle age adremial deficiency, perhaps combined with a quieter amid more settled life, may provide a more favorable environment for the multi plicationi of latent tumor cells. Other factors that may affect the situation innparticular tissues are the mitogemmic hormones and the poietinms. If the theory that one function of a mitogenic hormone is to neutralize the chalone of its target tissue is correct therm the resulting mitotic stimulus may be more powerfully felt in any latent tumor cells that are producing less than the normal amount of chalonc or reacting less adequately to whatever chalonme is present. The problem of hormone dependemit tumors amid of the progressive changes that occur in them has recently been discussed by Foulds (170, 171). While a tumor remains responsive to a hormone, it may “growso long as an appropriate (hormone) stimulus is maintained, regress when it is withdrawmi arid recur, at the same place and with the same characters, when it. is restored (170).― This is, of course, exactly what would be expected of armygroup of cells which arc omilymoderately damaged amid which maintain just sufficient control to limit their owmi growth. The hormone would theni act rather in the mariner of a promoting agent, increasing mitotic activity and reducing maturation, but when the hormomie is withdrawn these processes would be reversed amid the tumor might even regress com@)letcly. When, however, progressive chamiges towards greater malignancy occur, sonic of the cells might become so free from the normal mitotic control that they no longer would riced the hormone stimulus and ultimately might even lose all their ability to react to the hormone. By contrast, it seems improbable that the poictinis, which may not affect the mitotic rate, play any significant part in the cancer i)rOblcm. Theoretically a breakdown in mitotic amid functional homeostasis may occur in the progenitor cells of the crythrocytic, granulocytic, arid even the plasma cell systems, but these cells arc riot af fected by the poietimms. Alternatively the breakdowmi could occur in the relatively undifferentiated steni cells, 1715 amidin this case, too, the presence or absence of the poietins would be unlikely to exert any significant effect. V. GENERAL SUMMARY CONCLUSIONS AND The problem of mitotic horneostasis, with which this review began, is now seen to constitute only a part of the far larger 1)roblem of tissue homeostasis, which may be analyzed most profitably in terms of the control of genie expression. The programs of syntheses that lead to the creation and maimitenmance of a tissue are evidently de termined by a sequence of genie actions that are in turn determined by a sequence of humoral controls. There are at least. 3 main levels of these controls : the basic in duction mechanisms by which the tissues are formed, the chalone mechammisms by which they are maintained inn a functional state, arid in some cases the hormone mecha nisms by which tissue function may be modified. A. THE ROLE OF INDUCrNG SUBSTANCES There is a general belief that tissue induction is achieved through the initeractioni of an inducing sub.stanice with cells that arc conipetenit to react, and that the process involves the activation of omie region of the genonic arid the imiactivatiomi of most of the other regions. 1mmmum mals this inactivation may be pernianienit, arid innthe case of most tissues it. is completed in the embryo. However, it is also evident that in the adult mammal, with which this review is primarily concerned, (@crt.aingroups of stern cells, which retain at least part of their emiibryologic coniipetenice, are always present. These stem cells appear to belomig to 2 related groups, the 1st PerhaPs retaining only the dual potcnitiality of induction by poictinis to form either graniulocytes or erythrocyt.cs, amid the 2nd PerhaPs retaining the multiple potentiality of induction by antigens to form a variety of plasma cells. It is interesting that mieit.her the granulo cytic nor the erythrocytic cell PoPulations are regarded as self-mnainmtainiing in the manner of normal tissues since their rnit.otic potentialities are inisufficicmmtto meet their high rates of cell death. Thus innboth cases maintenmance of tissue mass depends on continued induction of the stem cells. 1mmthese unusual circunistanices it is Possible that. the tissue chalone mechanisms may be relatively poorly developed, but it is nevertheless clear that sonic consider able degree of chalomic control does exist arid that it. ninny also inifluemice the stem cell population. The organization of the lymphocyte-pla.srmia cell system is less well kniowmm. It. is l)rObablc that. it may contain the most complex systeni of interrelated tissues in the body, and that the details of its mitotic arid functional homeo static mechaniisms may be equally coniplex. However, it. would be sur@)risinmgif these mechanisms proved to in volve anything more thani variations on the 3 niaini types of homeostatic control. It has been rioted that. the graniulocytic, crythrocytic, and plasma cell systems are all tissues that must be able to meet sudden amid urgent denianids for large niunibers of extra cells. It. has been emphasized that such sudden tissue augmcnitationm is riot readily achieved through inn creased niitotic activity amid that. recruitment by iniduc 1716 Cancer Research tioni from the large stem cell populatiomis is a more efficient method. B. THE ROLE OF THE CHALONES It is evident that the creation of a tissue does not guarantee its continued functional efficiency and that at all times most mammalian tissues must be supported against the tendency of their cells to revert to a stable program of recurrent mitosis. It appears that, with in ductionm complete, the tissue cells normally retain at most only 3 groups of active or potentially active genes: the 1st concerned with the synthesis of general metabolic enzymes, the 2nd with tissue-specific function, and the 3rd with synthesis for mitosis. Together these groups of genes constitute the facultative genome, although it is possible that the 1st group may sometimes be nonfunc tional and that the synthesis of the general metabolic enzymes may be directed at the ribosome level. The 2nd amid 3rd groups are never fully functional simul taneously, and it now appears that the choice between tissue function and mitosis may be decided in terms of the intracellular concentration of the tissue-specific chalone. Thus the main task of the chalone systems of the body may be to maintain the whole functional organization of the animal against collapse into cellular anarchy. It is also evident that mitotic and functional homeo stasis is not a static affair; it responds to changing cir cumstances amid is subject to various checks and counter balances. The horneostatic mechanism continually maintains the tissue mass in correct relation to the body mass and reacts appropriately in conditions of tissue damage, whether local or general. Once the tissue mass is correctly related to the body mass, the situation is stabilized by the counterbalance provided by the inverse ratio between the mitotic rate and the life expectancy of the functional cells. Because of this, the point of balance between cell gain and cell loss is riot easily disturbed and tissue mass tends to remain constant. When the mitotic rate is greatly imncrea.sed, for instance by chalone lack through persistent cell damage, the tissue mass increases only moderately ; when the mitotic rate is reduced towards zero, the increased length of life of the functional cells is such as to prevent army change in the tissue mass. It is miot yet clear how the length of life of the functional cells may be imifluenced in this way. However, since nothing seems to affect the life expectancy of the non nucleated erythrocytes, it is possible that the time of cell death may be controlled at the gene level, possibly through the imntermediary of the lysosomes. The homeostatic mechanism is also responsive to the effects of stress. Adrenalin (but riot noradremialin) and the glucocorticoid hormones strengthen the action of the chalonies, and this, by reducing the rate of cell gain amid cell loss, may have sonic survival value, especially perhaps during starvation. C. THE ROLEOF THEHORMONES Froni time to time, in certain mammalian tissues, mitotic arid functional activity must be modified to meet particular needs, amid it is evident. that this must involve some initerferenice in the chalone mechanism. In the Vol. 25, November 1965 case of the hair growth rhythm such interference may be dependent on genes that oscillate spontaneously between activity and inactivity, but usually it seems to depend on the actions of diffusible substances that are synthesiaod in other @ti@sucsand may perhaps all be classified as hor mones. Of these the best known are the various mito genie hormones, such as the estrogens, which act to pro mote both mitosis and tissue function, perhaps through the neutralization of the chalones of the target tissues. When such tissues are unstimulated, it is evident that the mature cells are long-lived and nonfunctional ; when they are stimulated by chalone neutralization, the raised mitotic rate is accompanied by a reduced life expectancy of the mature cells, which become functional as they pass towards death. It is important to recall that such mitogenic hormones as the estrogens are widespread throughout the animal and plant kingdoms in situations in which their functions must be radically different from those they exert in an adult mammal. It seems indeed that certain types of molecules, which possess a pronounced ability to interfere in biochemical reactions, may have been incorporated in an opportunist manner in the control mechanisms of a wide variety of cellular activities. In these cases it is clearly the mechanisms that have evolved while the active substances have changed little. Although at the moment only relatively few hormones are known to be active in mammals as part of this 3rd order of- tissue control, it seems probable that a wide variety of other active substances may also be involved. These may perhaps include, for instance, promine and retine (470), the nerve growth factor (314), and the skin factor (119, 120), all of which have been omitted from the present discussion because they form such a hetero geneous group. Indeed, it appears that this 3rd order of tissue control may prove to be mediated through the ac tivities of such a varied assortment of substances that there may be little conimomi pattern except in their ulti mate disturbance of the chalone mechanism. It may be emphasized that many adult hormone mecha nisms are so organized as to regulate tissue functions terms of seasonal or other environmental changes. in D. THE PROBLEM OF GENE DAMAGE If all levels of mammalian tamed through the activities tissue organization of particular are main substances which, directly or indirectly, control the expression of the genome, then some at least of these substances may be regarded as acting in the traditional manner of the effec tors of the microorganisms. In these organisms versa tility in the face of a changing environment is obtained at the gene level by the variety of the possible alternative synthetic programs that may be activated by a wide range of effectors. In the metazoans such versatility is cvi dently obtained by the variety of the restricted genomes represented by the various tissues, amid in this situation the part played by effectors is less obvious. In this con nection it is important to attempt to determine how re stricted the geniome of a typical tissue may be. Of the possible syntheses a cell may undertake, it has already been mentioned that those relating to the general meta BuLL0uGH—Mitotic and Functional bolic enzymes may perhaps sometimes be directed by long-lived messenger RNA, but those relating to tissue function and to mitosis, being effector-cont.rolled alt.erna tives, must be directed at the gene level. 6. Alov, (532) and in mneurones.In this situation cell replaceniemit is impossible and molecular replacement is directed at. the ribosome level ; the accumulation of molecular damage at this level may cause amiincreasimig burden of malfunctionm. nonfunction, and even cell death, but it cannot lead to the rapid death of the animal itself through unregulated growth by mitosis. In most tissues, for a variety of obvious reasons, it is necessary to replace cells rather than molecules, amid the possibility of genie damage leading to cancer may be the price that such tissues must pay for the retention of a facultative genome capable of directing synthesis for mitosis. It is an indication of the homeostatic of the mechanism remarkable efficiency that this price is so rarely paid in the tissues of wild animals; that it is more often paid in the tissues of domestic animals be directly related to their relatively stressful lives. amid of niani may longer and less ACKNOWLEDGMENTS The ideas here put forward have emerged during a long study of mitotic control in adult mouse tissues, a study that would never have been possible but for the continuous and generous financial support of Organmon Laboratories Limited. These ideas have also been tested and often radically modified in the course of innumerable discussions, especially with Dr. E. B. Laurence, I)r. W. J. Tindall, Dr. C. L. Hewett, and Dr. L. Foulds in London, with Dr. J. I). H. Homan, Mr. H. de Jager, and Dr. W. Hondius Boldingh in Holland, and with Professor H. Teir and l)r. T. Rytomaa in Helsinki. Later, supported by a grant from the Damon Runyon Memorial Fund for Cancer Research, New York, long and fruitful discussions with Dr. Rytomaa were continued in London. Finally, the stimulus to consider the possible con nection between these new biologic theories and carcinogenesis was strengthened by an invitation to attend the Fiftieth Aniniver sary Meeting of the Netherlands Cancer Institute in Amsterdam. For all this varied assistance I am deeply grateful. I. A. 1)aily lation Adult Mammal. 2. . Behavior Symp. Soc. Exptl. Biol., 11: 235—54,1957. of Cells toward One Another. In: W. Mon tagna and R. Billingham (eds.), Advanmcesin Biology of Skin, Vol. 5, pp. 95—112.London : Pergamon Press, 1964. 3. Abercrombie, M., and Harkniess, R. I). The Growth of Cell Populations and the Properties in Tissue Culture of Re generating Liver of the Rat. Proc. Roy. Soc. (London), 5cr. B, 138:544—61, 1951. 4. Adibi, S., Paschkis, K. E., and Cantarow, A. Stimulation of Liver Mitosis by Blood Serum from Hepatectomized Hats. Exptl. Cell Res., 18: 396—98,1959. 5. Allfrey, V. G., Littau, V. C., and Mirsky, A. E. On the Role of Histones in Regulating Ribonucleic Acid Synthesis in the Cell Nucleus. Proc. Natl. Acad. Sci. U.S., 4.9: 414—21, 1963. Rhythm of Erythropoiesis Autoradiography. Cellular of Mitosis and Relationship and on Cellular In: F. Stohlman Proliferation, pp. l)ynamics (ed), be Proc. by Fe5' The Kinetic8 290—300. New York: Stratton,Inc.,1959. 8. Amoore, J. E. Arrest of Mitosis by Oxygen-Lack of Grunme & or Cyanide. Roy. Soc. (London), Ser. B, 154: 95—108,1961. . l)ependence of Mitosis and Respiration in ithots Oxygen Tension. Ibid., 154: 109—29, 1961. 9. upon 10. . Oxygen Tension and the Rates of Mitosis and Inter phase 11. in Roots. J. Cell Biol., @—. Participation 13: 365—71,1962. of a Non-Respiratory Ferrous Corn plex during Mitosis in Roots. Ibid., 13: 373—81,1962. 12. . Action Spectrum of Mitotic Ferrous Complex. Na ture,199:38—40, 1963. 13. . Non-Identical Mechanisms of Mitotic Arrest by Respiratory Inhibitors inn Pea Root Tips Eggs. J. Cell Biol., 18: 555—67, 196a. 14. Andersoni, M. H. Variations and Sea Urchin in the Hate of Induction of Chemical Carcinogenesis according to l)iffering Psycho logical States in Rats. Nature, @O4:55-56, 1964. 15. Andrews, E., and Hrdina, L. The Cause of l)eath in Liver Autolysis. 16. Anthony, Surg. Gynecol. Obstet., 52: 61-66, 1931. A., Akerman, E., and Lloyd, J. A. Noise Laboratory Rodents. I. Behavioral Stress and Endocrine inn Response of Mice, Rats, arid Guinea Pigs. J. Acoust. Soc. Am., 31: 1430—37,1959. 17. Argyris, T. S., and Argyris, B. F. Factors Affecting the Stirn ulation of Hair Growth during Wound Healing. Anat. Record, 14@: 139—45,1962. 18. Aronow, L., arid Gabourel, J. I). 1)eveloprnent of a Hydrocor tisone-resistant Sub-line of a Mouse Lymphoma in Vitro. Proc. Soc. Exptl. Biol. Med., 111: 348—49,1962. 19. Astarabadi, tomy arid T. The Effect of Hypophysectomy, Adrennalec ACTH Administration on Compensatory Renal Hypertrophy in Rats. Quart. J. Exptl. Physiol., 1963. 20. Baciu, J. La régulationm nerveuse et humorale poièse. J. Physiol., 54: 441—58, 1962. 48: 80-84, de l'érythro 21. Bade, E. G., and Llanos, J. M. E. Variation in the Mitotic Activity of the Liver during the Second 1)ay of Posthepatec tomy Regeneratiorm. Naturwissenischaften, @:693—94, 1963. 22. Barney, H. N., Vazquez, J. J., arid Dixon, F. J. Cellular Pro liferation inn Relation to Antibody Synthesis. Proc. Soc. Exptl. Biol. Med., 23. Barnium, 109: 1—4,1962. C. P., Jardetzky, C. I)., Acid Synthesis in Regenerating Mcd., 15:134—47, 1957. and Liver. Halberg, F. Nucleic Texas Rept. Biol. 24. Barr, G. C., and Butler, J. A. V. Histones and Genie Func tion. Nature, 199: 1170—72, 1963. 25. Barski, G. Recent Acquisitions in Malignant Transformation of Animal Cells in Vitro. Folia Biol., 9: 323—28, 1963. 26. —@—. Genetic Transformatiorms annd Interactions in in Vitro Somatic Cell Cultures. bock REFERENCES 1. Abercrombie, M. Localized Formation of New Tissue in ann 1717 tween Cell Work arid Cell Division. Federation Proc., 5@ (Transl. Suppl.): 357—62,1963. 7. Alpen, E. L., and Cranmore, 1). Observations on the Hegu It is evident that during life damage may accumulate at both the DNA amid long-lived RNA levels amid that by middle age such damage may be widespread. It follows that the fewer the number of active or potentially active DNA and RNA molecules that remain in a cell, the less is the chance that this accumulated damage will have serious consequences. Indeed the greatest tissue stability may be achieved through a finmalblockage of the emmtire genome, which possibly occurs both in striped muscle Homeostasis (eds.), Cellular In: P. Ernmelot and 0. Mühl Control Mechanmisms and Cancer, pp. 52—59. Amsterdam: Elsevier Press, Inc. 1964. 27. Bartter, F. C., 1)elea, C. S., and Halberg, F. A Map of Blood and Urinary Changes Related to Circadian Variations in Adrenal Cortical Function in Normal Subjects. Anti. N. Y. Acad. Sci., 98: 969—83,1962. 28. Baserga, H., and Shubik, P. The Action of Cortisone on Transplanted and Induced Tumors inn Mice. Cancer Res., 14: 12—16, 1954. 29. Becker, A. J., McCullough, E. A., and Till, J. E. Cytological Demonstration of the Clonal Nature of Spleen Colonies 1)erived from Transplanted 197: Mouse Marrow Cells. Nature, 452—54, 1963. 30. Belanger, L. F. Increased Mitotic Activity in the Glandular Stomach of the Rat following Hypophysectomy. Endocri nology, 66: 886—88, 1960. 31. Benitez, L., and Shaka, J. A. Cell Proliferation innExperimen Cancer Research 1718 tal Hydronephrosis and Compensatory 56. Brown, Renal Hyperplasia. Mice. 33. D. A. Growth Cancer of Sarcoma Res., 22: 180 in Adrenalectomized on the Growth 35. of Carcinogenesis. of Cocarcinogenic 1: 807—14, 1941. Action Action and its Significance of Skin in the Understanding Carcinnogenesis. holme and M. O'Connor In: 1963. of 59. Brues, A. M., and Marble, B. B. An Analysis of Mitosis in Liver Restoration. J. Exptl. Med., 65: 15-27, 1937. 60. Bucher, N. L. R. Regeneration G. E. W. Wolsten of the Genital Tract Epithelia innthe Rat. Acta Anat. of the Mouse following Local J. Anat., 89: 124—31, 1955. P. B. Conitracture Corntracture Rabbits' annd the Phenomenon Skin. Nature, Roy. and Wounds of Hair Neogenesis 177: 791—92, 1956. following Intra-peritoneal Injections of Liver Homogenates on the Microbiol. Scand., Acta Pathol. Suppl. 121, pp. 1-65, 1957. 66. the Cornea and Intestinal Mucous Membrane Exptl. Biol. Med., 45: 104—8,1958. of Rats. Bull. 49. Boutwell, R. K., Brush, M. K., and Rusch, Physiological Effects Associated with Chronic strictiorn. Am. J. Physiol., 154: 517—24, 1948. within F. Humoral Factors in Erythro poiesis. In: L. Tocantins (ed), Progress in Hematology. New York: Grune & Stratton, Inc., 1959. 52. Breedis, C. Regeneration of Hair Follicles aind Sebaceous (ilannds from Epithelium of Scars inn Rabbit. Cancer Res., 14: 575—79, 1954. 53. Briggs, R., and King, T. J. Changes in the Nuclei of Differen Endoderm Cells as Revealed by Nuclear J. Morphol., 100: 269—311, 1957. in the Adult Female Mouse, 5cr. B, @31 : 453—516, 1946. . Mitotic Activity inn the Adult Male Mouse, Mus musculus L. The l)iurnal Cycles and Their Relation to Wak Sleeping. Proc. Roy. Soc. (London), 5cr. B, 135: . The Effect of a Restricted Diet on Mitotic Activity in the Mouse. Brit. J. Cancer, 3: 275—82,1949. . Age and Mitotic Activity in the Male Mouse. J. I. The Effects fectsof the Sex Hormones.Ibid., 8: 365—76, 1952. 70. 71. @—.The Energy Relations of Mitotic Activity. Biol. Transplan of the Tonofibrils in the Kerat Human Epidermis. . A Study of the Hormonal Relations of Epidermal Mitotic Activity in Vitro. III. Adrenalin. Exptl. Cell Res., 9: 108—15,1955. . Vertebrate Reproductive Cycles. London : Methuen & Co., Ltd., 1961. 73. — . Tissues. 74. -@-@ . Nature, 75. . The Control of Mitotic Activity in Adult Mammalian Biol. Rev., 37: 307—42, 1962. Analysis of the Life-Cycle inn Mammalian Cells. 199: 859-62, 1963. Growth Regulation by Tissue-specific Factors, or J. Ultra Control Polynnuclear Aromatic Hydrocarbonns to the Nucleic Acids of Mouse Skin : Relation betweenn Carcinogenic Power of Hydrocarbons and Their Binding to l)eoxyribonucleic Acid. Mechanisms and Cancer, pp. 124—45. Amsterdam: Elsevier Press, Inc., 1964. 76. Bullough, W. S., and Ebling, F. J. Cell Replacement inn the Epidermis and Sebaceous Glands of the Mouse. J. Anat., 86: 29—34,1952. 77. Bullough, W. S., and Eisa, E. A. The Effects of a Graded Series of Restricted Diets orn Epidermal Mitotic Activity in the Mouse. Brit. J. Cancer, 4: 321—28, 1950. 78. Bullough, W. S., Hewett, C. L., and Laurence, E. B. The Epidermal Chalone : A Preliminary Attempt at Isolation. Exptl. Cell Res., 36: 192—200, 1964. 79. Bullough, W. S., and Johnson, M. The Energy Mitotic Activity in Adult Mouse Epidermis. Soc. (London), 5cr. B, 138: 562—75, 1951. Relations of Proc. Roy. 80. Bullough, W. S., annd Laurence, E. B. The Mitotic Activity of the Follicle. In: W. Montagna and R.. A. Ellis (eds.), The Biology of Hair Growth pp. 171—87. London : Academic Press, Inc., 1958. 55. Brookes, P., and Lawley, P. D. Evidence for the Binding of 202: 781-84, 1964. Activity Chalones. In: P. Emmelot and 0. Mühlbock (eds.), Cellular the Chick Brain. J. Morphol., 108: 263—85, 1961. Nature, of Incor of the Adrenmal Hormones. J. Endocrinol., 8: 265—74, 1952. 69. —@. Stress arid Epidermal Mitotic Activity. II. The Ef H. P. Some Caloric Re M. H. Regional Specificity of Inhibition inizationn Process of Normal struct. Res., 4: 264—97,1960. Rate Exptl. Biol., 28: 261—86,1949. 68. . Stress and Epidermal Mitotic Activity. 72. 47. Blumenthal, H. T. The Nature of Cycle Variations in Mi totic Activity : The Relation of Alimentation and Nutri tion to this Phenomenon. Growth, 14: 231—49, 1950. 48. Bogoyavlenskaya, N. V. , and Dobrokhotov, V. N. The Effect of Adrenalinne onn Mitosis, Respiration and Glycolysis in 54. Brody, I. The Ultrastructure M. M. The Rev., 27: 133—68, 1952. I)evelopment tiatinig t.ation. Soc. (London), 67. 46. Blomqvist, K. Growth Stimulation in the Liver arid Tumour 51. Brecher, G., and Stohlman, W. S. Mitotic ing and Cell Res., 30: 311—21,1963. 50. Braverman, 11: 457-65, 212—33, 1948. 65. —. The Effects of Experimenmtally Induced Rest and Ex ercise on the Epidermal Mitotic Activity of the Adult Male inn ties. New Enngl. J. Med. , P138: 477—80,539—45; 1963. 45. Block, P., Seiter, I., and Oehlert, W. Autoradiographic Studies of the Initial Cellular Response to Injury. Exptl. @- Res., Mouse, Mus musculus L. Ibid., 135: 233—42, 1948. Wound 43. Billingham, H. E., and Silvers, W. K. The Melanocytes of Mammals. Quart. Rev. Biol., $5: 1—40,1960. 44. . The Origin amid Conservationm of Epidermal Specifici Rat. Cancer Mum musculwr L. A Study of Its Relation to the Oestrous Cycle in Normal and Abnormal Conditions. Phil. Trans. 64. 98, 1969. H. E., and Medawar, Rats. N. L. H., and Swaffield, 63. Bullough, Oestro Initussusceptive Growth in the Healing of Extensive inn Mammalian Skins. J. Anmat., 89: 114—23, 1955. 42. Billingham, H. E., anid Russell, P. S. Incomplete in Parabiotic poration of Labeled Thymidine into the Deoxyribonucleic Acid of Regenerating Rat Liver in Relation to the Amount of LiverExcised.Ibid.,@4:1611—25, 1964. 40. Billinigham, R. E., Marigold, R., and Silvers, W. K. Neogene sis of Skin in Antlers of Deer. Ann. N. Y. Acad. Sci., 83: 491— 41. Billingham, Liver 62. Bucher, 54: 39—81, 1963. 39. Biggers, J. D., and Claringbold, P. J. Mitotic Activity in the Vaginal Epithelium genic Stimulation. Liver. Intern. 1951. Cytol., 13: 357—66,1962. . Mitotic Rates, Renewal Times, anmdCytodynamics of the Female of Mammalian Rev. Cytol., 15: 245-300, 1963. 61. Bucher, N. L. R., Scott, J. F., and Aub, J. C. Regeneration (eds.), Ciba Found. Symp. Carcino genesis, pp. 55-65. London: J. & A. Churchill, Ltd., 1959. 37. Bertalanify, F. D., and Lau, C. Cell Renewal. Intern. Rev. 38. Chreni@ Myeloid Leukemia. Advan. Cancer Res., 7: 351—81, Related the Mechanuism of Carcinogenesis. Ibid., 14: 471—77,1954. 36. . Some New Implications of the Two-Stage Mechanism in the Study of Intestinal Epithelial Cell Renewal and Migration Induced A Study and . A speculative Review : The Probable Nature of Pro moting and by Starvation. Am. J. Physiol., @O5: 868—72,1963. @8.Brown, W. M. C., and Tough, I. M. Cytogenetic Studies in of 3,4,9,10-Dibenzpyrene-induced I. The Mechanism of the Significance Phenomena. Ibid., in Normal 1962. Tumors. Ibid., 25: 1016—20, 1963. 34. Bereniblum, of Leukocytes 57. Brown, H. 0., Levine, M. L., and Lipkin, M. Inhibition 1220—25,1962. . The Effects of Adrenalectomy and Pyridoxine Dc ficiency H. E. Periodicity 1965 Adrenalectomized Mice. Ann. N. Y. Acad. Sci., 98: 995—1006, Am. J. Pathol., 44: 961-72, 1964. 32. Beniton, Vol. 25, November 81 . —@ . The Control of Epidermal Mitotic Activity in the Mouse. Proc. Roy. Soc. (London) 5cr. B, 151: 517—36,1960. 82. . The Control mis and Hypodermis. of Mitotic Activity in Mouse Skin. Der Exptl. Cell Res., 21: 394—405, 1960. BuLL0uGH—Mitotic and Functional 83. 1719 . Stress and Adrenaline inn Relation to the Diurnal Cycle of Epidermal Mitotic Activity in Adult Male Mice. 111. Cattaneo, S. M., Quastler, H., arid Sherman, F. G. Prolifera tive Cycle in the Growing Hair Follicle of the Mouse. Nature, Proc. Roy. Soc. (London) 5cr. B, 154: 540—56, 1961. 190:923—24, 1961. 112. Chase, H. B. Growth of the Hair. Physiol. Rev. , 34: 113—26, 84. . The Control of Mitotic Activity in the Skin. In: D. Slome (ed), Wound Healing, pp. 1—9.London : Perga 1954. 113. Chase, H. B., arid Eaton, G. J. The Growth of Hair Follicles mon Press, 1961. 85. . The Study of Mammalian Epidermal Vitro. A Critical Analysis of Technique. Exptl. Mitosis in Cell Res., inn Waves. 86. Soc. 87. the Chaloiie-Adrenalinn Complex. Exptl. of Epidermal the Chalone-Adrenalin Mitosis Symp. : the Zool. Role in Vitro : Effect Complex and of Energy Productions. 4:291—305, 1950. . Carcinogenesis and Cancer 1145—51, 1963. 94. Burnnet, F. M. Cancer—A Prevention. Biological Approach. Ibid., 197: Brit. Med. J.,i:779—86, 1957. 95. . The Inntegrity of the Body. Loridoni : Oxford Univer sity Press, 1962. 96. . A 1)arwiniiann Approach to Immunity. Nature, 203: 451—54,1964. 97. Burrows, H. Biological Actions of Sex Hormones. London: Cambridge University Press, 1949. 98. Burton, A. F. The Binding arid Metabolism of Cortisol-4- C'4 by Lymphatic Tissue and Tumors in Rats and Mice. Cancer Res., 24: 470—74, 1964. 99. Busch, H., Steele, W. J., Hinilica, L. S., Taylor, C. W., and Mavioglu, H. Biochemistry of Histonies and the Cell Cycle. J. Cellular Comp. Physiol., 62 (Suppl. 1): 95—110, 1963. 100. Cameron, G. H. Pathology Boyd, Ltd., 1952. of the Cell. Edinburgh: Oliver & 101. Cameron, G. H., Hassann,S. M., arid Dc, S. N. Repair of Glis soil's Capsule after Tangential Wounds of Pathol. Bacteriol., 73: 1—10, 1957. 102. Cameroin, G. H., arid Oakley, C. L. Ligation Bile 1)uct. Ibid.,35: 769—98, 1932. the Liver. of the J. Common 103. Cameron, I. L. Is the Duration of i)NA Synthesis in Somatic Cells of Mammals arid Birds a Conistant? J. Cell Biol., 20: 185—88,1964. 104. Cameron, Relation Chickens. I. L. , and Cleffmainrn, U. Initiation of Mitosis iii to the Cell Cycle followiing Feeding of Starved Ibid., 21: 169—74,1964. 105. Carnellakis, E. S., Jaffe, J. J., Mantsavinnos, H., arid Krakow, J. S. Pyrimidinne Metabolism. IV. A Comparisonn of Normal and Regenerating Rat Liver. J. Biol. Chem., 234: 2096—99, 1959. 106. Cainzaiielli, A. , (.uild, It. , arid Rapport, 1). Pituitary arid Adrenno-Cortical Relationships to Liver Regeneration arid Nucleic Acids. Endocrinology, 45: 91—95, 1949. 107. Carlsen, E. N., Trelle, G. J., and Schjeide, 0. A. Trainsfer Ribonucleic Acids. Nature, 202: 984—86, 1964. 108. Carnot, P., and May, R. M. La régénerationdu rein chez le rat btudiée au moyen de Ia coichicine. Compt. rend. Soc. Biol.,128: 641—43, 1938. 109. Cater, D. B., Holmes, B. E., arid Mee, L. K. Cell 1)ivisionn and Nucleic Acid Synthesis in the Regenerating Liver of the Rat. Acta Radiol., 46: 655—67,1956. . The Effect of Growth Hormone upon Cell Division arid Nucleic Acid Synthesis inn the Regenerating Rat. Biochem. J., 66: 482—86, 1957. Liver of the in Pinnial Epidermis Cortisol 117. 118. 120. J. J. Epineplirine of the Mouse. J. innPirnna Epidermis of or 9-a-Fluorocortisol. Biol. Med., 91: 602—4,1956. 116. Christensen, B. G., arid Jacobsen, 119. 90. Bullough, W. S., arid R.ytomaa, T. Mitotic Homeostasis. Nature, 205: 573—78, 1965. 91. Burch, P. H. J. A Biological Principle and its Converse: Some Implications for Carcinogenesis. Ibid., 195: 241—43, 1962. 92. . Humamn Cancer : Menidelian Inheritance or Vertical Transmission. Ibid., 197: 1042-45, 1963. 93. Mice Given of Ibid.,35: 629—41, 1964. 89. Bullough, W. S., arid Oordt, G. J. vani. The Mitogennic Ac tions of Testosteronne Propionate and of Oestrone on the Epidermis of the Adult Male Mouse. Acta Endocrinol., Activity Appl. Physiol., 9: 265—67,1956. 115. @—. Reduction of Mitotic Activity of Cell Res., 33: 176— Sci., 83: 365—68, 1959. A. P., Halberg, F., arid Bittner, arid Mitotic . The Production of Epidermal Cells. London, 12: 1—23, 1964. . Mitotic Control by linternal Secretioin 94, 1964. 88. . 1)uration Anus. N. Y. Acad. 114. Chaudry, @4: 289—97, 1961. 110. Homeostasis Proc. Soc. Exptl. E. Studies on Liver He generation. Acta Med. Scarnd., Suppl. 234, pp. 103—8,1949. Christie, G. S., and Judah, J. 1). Mechariisnn of Action of Carbons Tetrachloride on Liver Cells. Proc. Roy. Soc. (Lois don) Ser. B, 142: 241—57, 1954. Cleffmann, G. Functionm-specific Changes iii the Metabolism of Agouti Pigment Cells. Exptl. Cell Hes. , 35: 590—600, 1964. Cohen, S. Isolation of a Mouse Submaxillary Gland Proteins Accelerating Incisor Eruptiorn arid Eyelid Opening in the New-born Animal. J. Biol. Chem., 237: 1555—62, 1962. . Isolation arid Biological Effects of an Epidermal Growth-stimulating Protein . Natl . Cancer Inst . Monograph No. 13, pp. 13—21,1964. 121. Cooper, iii the E. H. Production Rat following of Lymphocytes Immunization with arid Plasma 1-lumars Serum Cells Albu mirn. Immunology, 4: 219—31, 1961. 122. Craddock, C. U. Production arid 1)istribution of Graniulo cytes arid the Control of Granulocyte Release. In: G. H. W. Wolsteriholme arid M. O'Connor (eds.), Haemopoiesis. Cell Production arid its Regulation. Ciba Found. Symp., pp. 237— 61. London: J. & A. Churchill, Ltd., 1960. 123. — . The Physiology of Grarnulocytic Cells irs Normal arid Leukemic States. Am. J. Med., 28: 711—25, 1960. 124. . The Production, Utilization arid I)estructiori of White Blood Cells. In: L. M. Tocanitis (ed), Progress irs Hematology, \ol. 3. New York: Grunne & Stratton, Inc., 1962. 125. Cruicksharik, C. N. 1)., Hershey, F. B., and Lewis, C. Iso citric 1)ehydrogenase Activity in Human Epidermis. J. Invest. l)ermatol., 30: 33—37,1958. 126. Curtis, A. S. U. The Cell Cortex. Endeavour, 22: 134—37, 1963. 127. Curtis, H. J. Biological Mechanisms Underlying the Aging Process. Science, 141: 686—94, 1963. 128. l)ales, S. Effects of Anaerobiosis on the Hates of Multiplica tioni of Mammalian Cells Cultured in Vitro. Cain. J. Biocineni. Physiol., 38: 871—78, 1960. 129. 1)avidsoni, E. H., Allfrey, V. (., arid Mirsky, A. H. Gene Expression iii l)iffereritiated Cells. Proc. Nati. Acad. Sci., 49: 53—60, 1963. 130. 1)avis, N. S. l)ysfunnction of Thiyroid, Pituitary, arid Adre rial Glands. In: E. J. Stieglitz (ed), Geriatric Medicine. Philadelphia: W. B. Saunders Company, 1943. 131. 1)eann, A. C. H., and Hinshelwood, 0. M. Sonic Basic Aspects of Cell Regulation. Nature, 201: 232—39, 1964. 132. 1)eferndi, V. , and Manisoni, L. A. Analysis of the Life-Cycle irs Mammalian Cells. Ibid., 198: 359—fl!, 1963. 133. l)eHaann, H. L., arid Ebert, J. 1). Morphogeriesis. Ann. Rev. Physiol., 26: 15—46,1964. 134. 1)emerec, M., arid Hartmani, P. E. Conmplex Loci in Microor gannisms. Ann. Rev. Microbiol., 13: 377—40(1,1959. 135. 1)obrokhotov, V. N. Regulations of Rhythmic Changes un Mitotic Activity inn Various Tissues of the Orgariisnn. Pathol. Biol.,9: 507—9, 1961. 136. . On the Importance of Regularities Governing thie l)iurnal Periodicity of Cellular Multiplication. Bull. Acad. Med. Sci.USSR., 18: 50—62, 1963. 137. 1)obrokhotov, V. N., and Kurdyumova, A. G. 24-Hour Periodicity of Mitotic Activity of the Epithelium inn the Esophagus of Albinio Rats. Bull. Exptl. Biol. Med., 53: 81— 84, 1962. 138. l)ohrokhotov, V. N., arid Nikaniorova, H.. I. 24-Hour l)aily Cancer Research. 1720 Periodicity of Cellular Mitosis in Adrenal Glands of Albino Rats. Ibid. , 54: 91—96, 1962. 139. 1)ougherty, T. F. Adrenal Cortical Control of Lymphatic Tissue Mass. In: F. Stohlman (ed), The Kinetics of Cellular Proliferation, pp. 264—74. New York: (.rune & Stratton, Inc., 1959. 140. Dribbeni, I. S., amid Wolfe, J. M. Structural 141. 142. 143. 144. 145. Changes Female Albino Rat. J. Endocrinol., 12: 38—49, 1955. 146. —-—. The Actions of Testosterone arid Oestradiol on the Sebaceous Glarnds arid Epidermis of the Rat. J. Embryol. Exptl. Morph., 5: 74—82, 1957. 147. . Hormonal Control of Sebaceous Glannds ins Experi mental Animals. In: W. Montagna, H. A. Ellis, arid A. F. Silver (eds.), Advances irs Biology of Skin, Vol. 4, pp. 200—19. New \ork : Pergamon Press, 1963. 148. Eblinig, F. J., arid Johnson, E. Systemic Influence on Ac tivity of Hair Follicles ins Skin Homografts. J. Embryol. Exptl. Morphol., 9: 285—93, 1961. 149. ——. The Control of Hair Growth. Symp. Zool. Soc. Lon don, 12 97—126, 1964. 150. Eigsti, 0. J., amid I)ustimn, P. Colchicine. Ames, Iowa: Iowa State University Press, 1955. 151. Erigel, L. L. Mechanisms of Actioni of Estrogens. Vitamins Hormones, 17: 205—22, 1959. 152. Enigelbreth-Holm, J., arid Asboe-Hariseni, G. Effect of Corti some on Skins Carcinsogenesis innMice. Acta Pathol. Microbiol. Scanid., 32: 560—64, 1953. 153. Epel, 1). The Effects of Carbon Monoxide Inhibitions onnATP Level arid the Rate of Mitosis in the Sea Urchin Egg. J. Cell 164. Fischberg, at \arious Stages of the Sexual Cycle of Mice. Bull. Exptl. Biol. Med., 46: 113—16,1958. 155. . Analysis of the effect of Repeated Oestrone Injections Oii 156. 157. 158. 159. 160. 161. 162 . the Mitotic Activity of Uterine Epithelium in Mice. ibid.,51: 102—5, 1961. . Possible Means of Hormonal Regulation of the Mi totic Cycle. Cytologia, 4: 128—36,1962. @—.Effect of Oestrone on Mitotic Cycles in Some Epi thelial Tissues inn Mice. Arch. Anat. Histol. Embryol., 46: 27—36,1964. Epifaniova, 0. I., arid Tchoumak, M. C. On the Actions of Adrenialins upon the Mitotic Cycle of the Intestinal Epi theliumu inn Mice. Cytologia, 5: 455—58, 1963. Eränkö,0., Karvoriens, M. J., and Räisännen,L. Long-Term Effects of Muscular Work on the Adrenal Medulla of the Rat. Acta Enndocriniol., 39: 285—87, 1962. Eskin, M. A., arid Vidavskaya, G. M. The Influence of Day arid Night on the Eosinophil Content of Peripheral Blood aind on the Function of the Adrenal Cortex. Probl. Endocri riol. Hormone Therap., 2: 82—87,1956. Euler, U. S. von. Noradrenialine. Springfield, Ill. : Charles C Thomas, Publisher, 1956. . The Catecholamines . Adrenaline ; Noradrenaline. In: C. H. Gray and A. L. Bacharach (eds.), Hormones Blood, pp. 515—82.Lormdon: Academic Press, Inc., 1961. 163. Fautrez, J., Cavalli, G., arid Pisi, E. Variations in Amounts in M., and Blackler, A. W. How Cells Specialize. Sci. Am., 205: 124—40,1961. 165. —@—.Loss of Nuclear Preservation of Nuclear Potentiality Potentiality inn the Soma Versus in the Germ Line. In: R. J. C. Harris (ed), Biological Organization at the Cellular and Supercellular Level, pp. 111—22. London: Academic Press, Inc., 1963. 166. . Nuclear Changes during the 1)ifferentiation of Ani mal Cells. Symp. Soc. Exptl. Biol., 17: 138—56, 1963. 167. Fischberg, M., Gurdons, J. B., and Elsdale, T. R. Nuclear Transfer in Amphibia and the Problem of the Potentialities of the Nuclei of Differentiating Tissues. Exptl. Cell Res., Suppl. 6, pp. 161—78,1959. 168. FITZGERALD, P. H. The Immunological Role arid Long Life Spain of Small Lymphocytes. J. Theoret. Biol., 6: 13—25,1964. 169. Formisano, V. H., and Montagna, W. Succiniic Dehydroge nase Activity in the Skin of the Guinea Pig. Anat. Record, 120: 893—906,1954. 170. Foulds, L. Some Problems of 1)ifferenntiatiors arid Instegra tionn iii Neoplasia. In: R. J. C. Harris (ed), Biological Or ganiization at the Cellular and Supercellular Level, pp. 229— 44. London : Academic Press, Inc., 1963. 171. —@. Tumour Progression amid Neoplastic 1)evelopmenst. In: P. Emmelot 172. 173. 174. 175. 176. 177. Biol., 17: 315—19,1963. 154. Epifariova, 0. I. Mitotic Regimen insthe Uterine Epithelium 1965 of I)eoxyribonnucleic Acid in Cell Nuclei and Its Correlations with Mitotic Activity : Compensatory Hypertrophy of Kid niey. Nature, 175: 684—85,1955. in the Connective Tissues of the Adrenal Glands of Female Rats Associated with Advancing Age. Anat. Record, 98: 577—86, 1947. i)urward, A., arid Rudall, K. M. Studies on Hair Growth irs the Hat. J. Aniat. , 83: 325—35, 1949. ———.The Vascularity arid Patterns of Growth of Hair Follicles. In: W. Monitagnna amid H. A. Ellis (eds.), The Biol ogv Of Hair Gro@s'th, pp. 189—218.New York: Academic Press, Inc.,1958. l)ustiri, A. P. Etude de l'hypertrophie compensatrice du reins par la reaction stathmocinétique. Acta Unnio Intern. Contra Canicrum, 4: 679—83, 1939. 1)ustini, A. P., arid Zylberszac, S. Etude de l'hypertrophie comperisatrice du rein par Ia reaction stathmocinétique. Bull. Acad. Roy. Med. Belg., 4: 315—20, 1939. Eblinig, F. J. Endocrine Factors Affecting Cell Replacement arid Cell Loss iii the Epidermis and Sebaceous Glands of the Vol. 25, November 178. and 0. Mühlbock (eds.), Cellular Control Mechanisms arid Cancer. Amsterdam: Elsevier Press, Inc., 1964. @—.Multiple Etiologic Factors in Neoplastic i)evelop merit.Cancer Res., 25: 1339—47, 1965. Franiseen, C. C., Brues, A. M., and Richards, R. L. The Effect of Hypophysectomy on the Restoration of the Liver F3llowinng Partial Hepatectomy in the Rat. Endocrinology, 23: 292—301,1938. Friedenwald, J. S., and Buschke, W. The Effects of Excite merit, of Epinephrine and of Sympathectomy on the Mitotic Activity of the Corneal Epithelium inn Rats. Am. J. Physiol., 141: 689—94,1944. Friedewald, W. F., amid R.ous, P. The Initiating arid Promot ing Elements in Tumor Production. J. Exptl. Med., 80: 101—44,1944. . The Pathogenesis of Deferred Cancer. Ibid., 91: 459—84, 1950. Friedrich-Freksa, H. von, arid Zaki, F. G. Spezifische Mitose Auslosunig inn nnormaler Rattenileber durch Serum von partiell hepatektomiertenn R.atten. Z. Naturforsch., B9: 394—97, 1954. Fry, H. J. M., Lesher, S., and Kohmn, H. I. Estimation of Time of Generations of Living Cells. Nature, 191: 290—91, 1961. 179. Fuller, H. H., Brown, E., and Mills, C. A. Environmental Temperatures arid Spontaneous Tumors in Mice. Cancer iles.,1: 130—33, 1941. 180. Fuxe, K., and Nilssons, 0. The Mouse Uterine Surface Epi thelium during the Estrous Cycle. Aniat. Record, 145: 541— 48, 1963. 181. Gabourel, J. 1)., arid Aroniow, L. Growth Inhibitory Effects of Hydrocortisone on Mouse Lymphoma ML-388 in Vitro. J. Pharmacol. Exptl. Therap., 136: 213—21, 1962. 182. Galicich, J. H., Halberg, F., arid French, L. A. Circadian Adrenal Cycle in C Mice Kept without Food arid Water for a i)ay and a Half. Nature, 197: 811—13, 1963. 183. Gavosto, F., Pileri, A., Bachi, C., and Pegoraro, L. Pro liferationn and Maturation Defect inn Acute Leukaemia Cells. Ibid., 203: 92—94, 1964. 184. Gelfanit, S. The Energy Requirements for Mitosis. Arm. N. Y. Acad. Sci., 90: 536-49, 1960. 185. Gel'shtein, V. I. Mouse Liver Regenieratiorn in the Process of Experimental Carcinogeriesis. Fed. Proc., 23 (Transl. Sup p1.): 358-61, 1964. 186. Gesner, B. M., and Gowanis, J. L. The Fate of Lethally Ir radiated Mice Given Isologous and Heterologous Thoracic Duct Lymphocytes. 187. Ghadially, Brit. J. Exptl. Pathol., F. N., and Green, 43: 431—40,1962. H. N. The Effect of Cortisone BuLL0UGH—Mitotic and Functional on Chemical Carcinogenesis in the Mouse Skin. Brit. J. Cancer, 8: 291—95, 1954. 188. Gillman, T., Hathorn, on Cutaneous M., and Penn, and Pulmonary J. Actions Neoplasms of Cortisone Induced in Mice by Cutaneous Applications of Methylcholanthrene. 10: 394—400,1956. 189. Gillman, T., and Penn, J. Studies on the Repair Ibid., of Cutaneous Wounds. Med. Proc. S. Africa, 2: 121—84, 1956. 190. Glinos, A. D. Mechanism of Growth Control innLiver Re generation. Science, 1@3:673—74, 1956. 191. . Inhibition of Liver Cell Division and Serum Proteins Changes Induced by Dehydration in Partially Hepatecto mized Rat. Federation Proc., 15: 76, 1956. 192. . The Mechanism of Liver Growth and Regeneration. In: W. D. McElroy and B. Glass (eds.), The Chemical of Development, pp. 813—39.Baltimore : Johns Hopkins 1958. 193. Basis Press, @-. Environmental Feedback Control of Cell Division. Ann. N. Y. Acad. Sci., 90: 592-602, 1960. 194. Glinos, A. I)., Bucher, N. L. R., and Aub, J. C. The Effect 196. Godefroy, F. Influence du jetine sur la teneur M. T. Changes in Mitotic en adrenaline Activity C. Innteractive Processes of Rats ins Cytodifferentiation. J. Cellular Comp. Physiol., 60: 35-48, 1962. 214. Grunidmanni, E. I)ie Bildung der Lymphocyteni unid Plas mazellen in lymphatischenn Gewebe der Ratte; eimncytolo gischer allgem. Beitrag Pathol., zur Blutzellreifung. 119: 217—62,1958. Beitr. Pathol. Annat. 215. Grunneberg, H. The Genetics of the Mouse. The Hague: Mar tinus Nijhoff,1952. 216. Guillemin, R., I)ear, W. E., arid Liebelt, A. Nychthemeral Variations inn Plasma-free Corticosteroid Levels of the Rat. Proc. Soc. Exptl. Biol. Med., 101: 394—95, 1959. 217. Gurley, L. R., Irvin, J. L., and Holbrook, 1). J. Inhibitions of DNA Polymerase by Histories. Biochem. Biophys. Res. Communs., 14: 527—32, 1964. 218. Gurney, C. W., Lajtha, L. G., arid Oliver, H. A Method for Investigation of Stem-Cell 8: 461-66, 1962. Kinetics. Brit.. J. 219. Guzek, J. W. Effect of Adrennocorticotrophic Cortisone et en noradrénalinedes surrenales et de l'urine. Compt. rend. Soc. Biol., 158: 693—96, 1964. 197. Gololobova, 213. Grobstein, J. Exptl. Med., 93: 313—24,1951. 195. Glinos, A. D., and Gey, G. 0. Humoral Factors Involved in the Induction of Liver Regeneration in the Rat. Proc. Soc. Exptl. Biol. Med., 80: 421—25, 1952. 1721 212. Grisham, J. W. Deoxyribose Nucleic Acid Synthesis and Cell Renewal in Regenerating Rat Liver. J. Histochem. Cytochem., 8: 330, 1960. of Regeneration on Tumor Formation in Rats Fed 4-Dimethyl Aminoazobenzene. Homeostasis @nithe generating Liver Uptake Tissue of Tritiated inn the White Haematol., Hormone Thymidimne Rat. Nature, and by 201: Re 930— 31, 1964. 220. Haddow, Physical A. Mechanisms arid Viral. Brit. 221. Hagemi, P. The Storage of Carcinsogenesis: Chemical, Med. Bull., 20: 87—90, 19(4. arid Release of Catecholaminies. Pharmacol. Rev., 11: 361—73, 1959. 222. Halberg, F. Depending on the Time of Day. Bull. Exptl. Biol. Med., Procedural 46: 118—22,1958. Cycle. Physiologic 24-Hour Considerations Z. Vitamin. Periodicity; General with Reference Hormoni. arid to the Adrenal Fermentforsch., 10: 225—96, 198. Gorbman, A. Thyroid Hormones. In: U. S. von Euler arid 1959. H. Heller (eds.), Comparative Endocrinology, pp. 291—324. 223. Halberg, F., Visscher, M. B., arid Bittnner, J. J. Eosinnophil London : Academic Press, Inc., 1963. Rhythm in Mice: Range of Occurrenice; Effects of Illuminsa 199. Gordon, A. S. Endocrine Influences ments of Blood and Blood-forming 200. 201. 202. 203. 204. 205. 206. upon the Formed Ele Organs. Recent Progr. tion, Feeding arid Adrennalectomy. Am. J. Physiol., 174: 109—22,1953. Hormone Res., 10: 339—87, 1954. 224. Hamburger, C. Normal Urinary Excretions of Neutral 17Gorski, J. Early Estrogen Effects on the Activity of Uterine Ketosteroids with Special Reference to Age arid Sex Varia Ribonucleic Acid Polymerase. J. Biol. Chem., 239: 889—92, tions. Acta Endocrinol., 1: 19—37,1948. 1964. 225. Hanawalt, P. C., Maalmøe, 0., Cummings, 1). J., arid Goss, R. J. Experimental Investigations of Morphogennesis Schaechter, M. The Normal 1)NA Replication Cycle. J. in the Growing Antler. J. Embryol. Exptl. Morphol., 9: Mol. Biol., 3: 156-65, 1961. 342—54,1961. 226. Harding, C. V, amid Srinivasari, B. I). A Propagated Stimu @—. The Deciduous Nature of I)eer Antlers. Am. Assoc. lations of 1)NA Synthesis and Cell l)ivisioni. Exptl. Cell Res., 25: 326—40,1961. Advan. Sci., Publ. No. 75, pp. 339-69, 1963. @—. Effects of Maternal Nephrectomy on Foetal Kidneys. 227. Harkness, H. 1). The Spatial l)istributions of l)ividing Cells Nature, 198: 1108—9,1963. inn the Liver of the Rat after Partial Hepatectomy. J. Phys Goss, R. J., and Rankin, M. Physiological Factors Affecting iol., 116: 373—79,1952. Compensatory Renal Hyperplasia inn the Rat. J. Exptl. 228. —-—. Regeneration of the Liver. Brit. Med. Bull., 13: 87— Zool., 145: 209—16, 1960. 93, 1957. 229. Harris, G. W. Neural Control of the Pituitary Gland. Lois Gowans, J. L. The Fate of Parental Strain Small Lympho cytes inn F1 Hybrid Rats. Ann. N. Y. Acad. Sci., 99: 432-F'S don : Edward Arnold & Co., 1955. 1962. 230. Harrison, H. G., amid Hoey, M. J. The Adrenal Circulations. Gowans, J. L., and Knight, E. J. The Route of Recirculationi Oxford: Blackwell Scientific Publications, 1960. 231. Hay, E. 1). Recent Studies of Embryonic Induction. New of Lymphocytes irs the Rat. Proc. Roy. Soc. (London), Ser Enigi. J. Med., 268: 1114—22, 1963. B, 159:257—82, 1963. 207. Gowans, J. L., arid McGregor, 1). 1). The Production of Lymphocytes by Lymphoid Tissue. In: L. F. Lamertonn arid R. J. M. Fry (eds.), Cell Proliferation, pp. 113—25. Oxford: Blackwell Scientific Publications, 1963. 208. Gowans, J. L., McGregor, 1). D., arid Coweri, 232. Hecht, L. I., arid Potter, V. Regenerating Rat Liver. V. arid ins Tissue Slices. Cancer 2.33. Hegyeli, A., McLaughlini, J. the Chemistry of the Thymus (.ilansd. Proc. Nati. Acad. Sci., 49: 230—32, 1963. I). M. The ROle of Small Lymphocytes in the Rejection of Homographs of Skins. In: G. E. W. Wolstenholme arid J. Knight (eds.), The Immunologically Competent Cell, pp. 20—29. London J. & A. Churchill, Ltd., 1963. 209. Gray, C. H., Greenaway, J. M., and Holniess, N. J. The Cor ticosteroids. In: C. H. Gray and A. L. Bacharach (eds.), Hormomnes irs Blood, pp. 439—514.Londoni : Academic Press, 234. Heilmami, Mammalian (.lrowth of a Malignnannt Tumor 34: 416—20, 1944. 235. Hell, E. A., arid Cruickshannk, upon 211. the Uptake —.Collagen arid Cell Protein Synithesis by annEstab lished Mammalian Fibroblast Line. Ibid., 204: 347—49, 1964. E. C. The of Influence (Conipounid in the Mouse. by Guinea on the Enidocrinnology, C. N. I). The Effect 3H-Thymidinie of 11-1)e- E) Pig of Injury Epidermis. Exptl. Cell Res., 31: 128—39, 1963. Cell Lines. Nature, 200: 1097—98, 236. Hemingway, iii 1963. @ F. It., arid Kendall, hydro-17-Hydroxycorticosteronne Inc., 1961. 210. Green, H., and Goldberg, B. Kinetics of Collagen Synthesis by Established H. Nucleic Acid Metabolism irs Comparisons of Results in Vivo lies., 18: 182—92,1958. A., arid Szenst-Györgyi, A. On J. T. Corticosteroids Regenerating Liver of the arid the Radiations Rat. . Experienitia, 15: Effect 189—90, 1959. 237. Withdrawal of Inhibition of l'ilitOr4is as a ?nIechannisni Cancer Research 1722 Influencing Normal arid Pathological Growth. Nature, 185: 106—7, 1960. 238. —@--—.Influence of Plasma Proteins in the Control of Mito sis-Rates inn Generatinig Liver. Ibid., 191: 706—7,1961. 239. Ilerlanit, M. ActivitC mitotique des cellules rCnale au cours J. Physiol., 119: 21P—22P, 1953. 266. Jaffe, J. J. Diurnal 13: 315—30,1948. 240. —@. Experimental Method. Hydromnephrosis Nature, 162: Studied 267. Jaffe, J. J., Fischer, 251—52, 1948. (Compound E) U@Oii a Lymphoid ibid., 245. Hoar, Mice. Leukemia Induced in irs (.uinsea 246. Hochstddt, Pigs. Ibid., Malformations 144: 155-64, B. B., arid Reichenibach, B. The Process of Ageimig 3: 55—62,1961. Marking of Cells innTwo 1)ifferenit Segments of the Mitotic Cycle. Na ture, A., arid Williams, T. L. The Influence of Exercise on the Rat Tumors. Cancer Res., 272. the Removal of Thymidine Kinase. Ibid., 49: 861—65, 1963. 252. Howard, A., arid Pelc, S. R. Synthesis inn Normal 274. Chromosome arid Irradiated Breakage. of Desoxyribonucleic Cells Heredity, and its Relation to Suppl. 6, pp. 261—73,1953. 254. Hughes, P. E. Hormonal Factors ins Liver Regeneration. Austral-asian Ann. Med., 9: 41—43,1960. 255. Hunt, T. E. Regerieratiorn of the Gastric Mucosa irs the Rat. Anat. Record, 131: 193—211,1958. 256. Hurowitz, H. B., arid Studer, A. Effect of Partial Hepatec tomy on Mitosis Rate in CC14-induced Liver I)amage of Rats. Arch. 257. Irvini, J. L.; holbrook, Pathol., 69: 511—15, 1960. W., H. C., Butler, J. A. V. Specificity of the Interac Wound Healing of Body Surfaces in Mammals. 35: 364—412, 1960. Biol. Rev., Kaplan, B. H. 275. Kärki, S., Marder, Cancer N. S. N., and Brown, M. Adrenal T. Res., The 11: 629—33, 1951. Urinary Excretion of Noradrenaline and Adrenaline in Different Age Groups, Its Diurnal Variation and the Effect of Muscular Work on It. Acta Physiol. Scand., 39 (suppl. 132): 1—96,1956. 276. Kessner, D. M., and Thomas, E. I). Renal Proliferation in the Unilaterally Nephrectomized Rat. Nature, 190: 175—76, 1961. 277. King, 1). W. Effect @1:1143—46,1962. 278. Kirk, J. E. Steroid of Injury on the Cell. Federation Proc., Hormones and Aging: A Review. J. Ger onitol., 6: 253—62,1951. 279. Klein, G., and Klein, E. The Evolution from Specific Growth Stimulation and of Independence Inhibition in Mammal ian Tumour-cell Populations. Symp. Soc. Exptl. Biol., 11: 280. 305-28, 1957. . Histocompatibility Changes in Tumours. J. Cellular Comp. Physiol., 52 (Suppl. 1): 125-68, 1958. 281. Knobil, E., arid Samidler, H.. The Physiology hypophyseal Hormones. In: U. S. von Euler (eds.), Comparative Endocrinology, demic Press, Inc., 1963. 282. Kochakian, I). J., Evans, J. H., McAllister, amid Cortical Function and Radiation-induced Lymphoid Tumors 253. Iluamig, H. C., arid Bonnnner, J. Histone, a Suppressor of Chro mosomal RNA Synthesis. Proc. Natl. Acad. Sci., 48: 1216—22, 1962. Parabiotic E. in Mice. . Molecular Facets of Mitotic Regulation. II. Factors Acid Johns, Action. Per tions between Histones and Deoxyribonucleic Acid. Nature, 204: 853—55,1964. 273. Johnson, F. R., and McMinn, It. M. H. The Cytology of 99, 1962. Underlying at 1963. 271. Jensen, E. V. On the Mechanism of Estrogen spectives Biol. Med., 6: 47—59,1962. 22 597— 249. Horvath, E. , arid Kovacs, K. Beitrage zur R.olle der Neben nniere inn der Regeneration der Leber. Z. Ges. Exptl. Med., 127:236—40, 1956. 250. Ilotta, V., arid Stern, H. Molecular Facets of Mitotic Regula tion. I. Synthesis of Thymidine Kinnase. Proc. Natl. Acad. Sci., 49: 648-53, 1963. 251. Rat . Histochemical and Degenerative Changes in the Adrenal Cortex of the Rat with Age. J. Gerontol., 12: 1—8, 1957. 270. . A Histo-cytologic Study of the Adrenal Cortex in K. E., 1)e Bias, D. A., Cantarow, of Transplanted A. D. Structure-Action 269. 202: 981—83, 1964. Growth G. A., and Welch, 268. Jayne, E. P. Cytology of the Adrenal Gland of the 1)ifferennt Ages. Anat. Record, 115: 459—83,1953. Pro 1962. arid Adreniocortical Activity. Geronstol. Clin., 247. Hof, J. vans't, amid Ying, H-K. Simultaneous 248. Hoffman, S. A., Paschkis, Rat Mice as Influenced by Strain, Sex, and Age. Ibid., 18: 227—34, of Congenital duced by Ilydrocortisonse to Those Produced by Adrenalec tomy in Regenerating Relationships of Corticosteroid Compounds as Inhibitors of Leukemic L5187Y Cell Reproduction in Vivo and in Vitro. Biochem. Pharmacol., 12: 1081—90,1963. 106: 204, 1950. H. M. Similarity Mitotic Periodicity Liver. Anat. Record, 120: 935—54, 1954. by the Colchi 241. Hieger, I. Theories of Carcinogenesis. In: G. E. W. Wolsten holme arid M. O'Connor (eds.), Ciba Found. Symp. Carcino genesis, pp. 3—11.London: J. & A. Churchill, Ltd., 1959. 242. . Carcinnogenesis. London: Academic Press, Inc., 1961. 243. Higgins, G. M. , amid Inigle, 1). J. Regeneration of the Liver in Hypophysectomized White Rats. Aniat. Record, 73: 95—104, 1939. 244. Higgins, (L M., amid Woods, K. A. The Influence of Cortisone AKM 1965 264. Jacobson, L. 0., Goldwasser, E., Fried, W., arid Plzak, L. Role of the Kidney in Erythropoiesis. Nature, 179: 633—34, 1957. 265. Jacoby, F. Mitotic Activity inn the Gall Bladder Epithehium of the Guinea-Pig after Ligation of the Common Bile Duct. de hydroniCphrose uriilatérale.Bull. Acad. Roy. Med. Beig., cine Vol. 25, November C. I)., arid Harrison, Acid Synthesis of the Adeno and H. Heller pp. 447—91.London, Aca D. G. Regulation by Arndrogens. Endocrinology, of Nucleic 70: 99—108, arid Stiles, E. P. Possible Role of Histories inn Regulationi of 1962. Nucleic Acid Synthesis. Exptl. Cell Res., Suppi. 9, pp. 359— 283. Kohmn, H. Effect of Administration of Rat Serum on Rat 66, 1963. Liver Regeneration. Exptl. Cell Res., 14: 228—30, 1958. 258. Isotalo, A., arid Teir, H. Influence of Cortisone on Mitosis. 284. Kondics, L. 1)iurnal Rhythm in the Adrenal of Albino Mice. II. Effects of Simultaneously Applied Cortisone arid Colchi cine. Ann. Med. Exptl. Biol. Fennniae, 259. Iversenn, 0. H. The Regulation of Cell Numbers innEpider mis. A Cybernetic Point of View. Acta Pathol. Microbiol. Scanid., Suppl. 148, pp. 91—96,1961. 260. Iversens, 0. H., arid Bjerknes, H. Kinetics of Epidermal Reac tiorn to Carcinnogenesis. Ibid., Suppl. 165, pp. 1—74,1963. 261. Jackson, B., arid Bohrnel, E. Effect of Serum Injections on Body Weight annd Liver Regeneration of Partially tomized Rats. Federation Proc., 21: 301, 1962. 262. Jacob, F., arid Moniod, Bacteria. J. Elements In: R. J. C. Harris at the Cellular of Regulatory Hepatec Circuits Level, pp. inn 1—24.London, Academic Press, Inc., 1963. 263. Jacobson, L. 0., arid l)oyle, M. (eds.). Erythropoiesis. New York: Grunne & Stratton, line., 1962. and RNA Synthesis during Mitosis inn Animal Cells. J. Cell Biol., 19: 267—77,1963. 286. Kramsch, I)., Beck, V., and Oehlert, W. Einfluss der Athio nninvergiftung und des Nahrungsenntzuges auf die DNS Neubildung inn den Wechselgewebenn und parenchymatosen Organemn der Ratte. Beitr. Pathol. Annat., 1S8: 416—44,1963. 287. Krasilnikova, N. V. Twenty-four-Hour Mitotic Activity in Reparative Regeneration of the Salivary Gland, Liver and (ed.), Biological Organization amid Supereellular Acta Biol. Acad. Sci. Hung., 1$: 265—71, 1962. 285. Konrad, C. G. Protein Synthesis 31: 301—4, 1953. Epidermis 99, 1963. of Albino Mice. Bull. Exptl. Biol. Med., 56: 96- 288. Kroeger, H. Chemical Nature of the System Controlling Genie Activities in Insect Cells. Nature, 200: 1234—35,1963. 289. Kursheva, A. N. Changes in Conditioned during Growth of Experimental Tumours Reflex Activity 1)epemnding on the BuLL0uGH—Mitotic and Functional Type of Nervous System. Bull. Exptl. Biol. Med., 45: 1—3, (ed), The Kinetics of Cellular Proliferation, pp. 173—82. New York: Grune & Stratton, 292. - . Erythropoietin Inc., 1959. and Cellular Kinetics in the Marrow. In: P. C. Williams (ed.), Hormones and the Kidney, pp. 239— 44. London : Academic Press, Inc., 1963. 293. Lajtha, L. G., Gilbert, C. W., Proteous, D. D., arid Alexan ian, A. Kinetics of a Bone-Marrow Stem-Cell Population. Ann. N. Y. Acad. Sci., 113: 742—52,1964. 294. Lajtha, L. G., and Oliver, R. Studies on the Kinetics of Erythropoiesis. A Model of the Erythron. In: G. E. W. Wol stenholme and M. O'Connor (eds.), Ciba Found. Symp. Ha emopoiesis, pp. 289—314. London: J. & A. Churchill, Ltd., 1960. 295. Lajtha, L. G., Oliver, R., and Gurney, C. W. Kinetic Model of a Bone-Marrow Stem-Cell Population. Brit. J. Haematol., 8: 442-60, 1962. 296. Lamerton, L. F. Radiation 20: 134—38, 1964. 297. Lampkin, J. McC., Carcinogenesis. Brit. Med. Bull., 313. Levi, L. The Urinary Output of Adrenalin and Noradrenalin during Experimentally Induced Emotional Stress in Clini cally Different 11: 218—27,1963. sis and Its Inhibition innMammalian Cells Cultured M. Response to Cortisone 82: 75-80,1963. 319. Llanos, J. M. E., arid Piezzi, H. S. Twenty-four Hour Rhythm in the Mitotic Activity of Normal Mammary Epithelium on Normal arid Inverted Lighting Regimens. J. Physiol., 165: 437—42,1963. 320. Llanos, J. M. E., arid Saffe, I. E. Accion de la regeneracioni tommie espontaneous and of the Mouse. J. Natl. Cancer J. McC. Response of a Localized Inst., Lympho cytic Neoplasm of the Mouse to 9a-Fluorohydrocortisonne and Related Steroids. Ibid., @4:1353—66,1960. 299. Lancker, J. L. van, and Borison, H. L. Incorporation of Tn from the Animal. J. Biol. Chem., @$7:1634—42,1962. 317. Llannos, J. M. E., and Badran, A. F. Ritmo de 24 horas en la actividad mitotica de un carcinoma mamario injertado de C3H/mza hembra. Rev. Soc. arg. biol., 37: 226—39,1961. 318. . 24-Hour Rhythm in the Mitotic Activity of a Grafted Mammary Carcinoma in Female C,H/mza Mice on Normal and Inverted Lighting Regimens. J. Roy. Microscop. Soc., Development of Cortisone Resistance in a Cortisone-sensi 298. Lampkin-Hibbard, Gvonp@. Atta Psychotherap., 314. Levi-Montalcini, R. The Nerve Growth Factor. Ann. N. Y. Acad. Sci., 118: 149—70,1964. 315. Levi-Montalcini, R., and Angeletti, P. V. Growth arid Dif ferentiation. Arm. Rev. Physiol., @4:11—56,1962. 316. Liebermann, I., arid Ove, P. Deoxyribonucleic Acid Synthe hepatica sobre la incidencia y caracteristicas arid Potter, tive Lymphosarcoma @O: 1001-08, 1958. 1723 Cycle innthe Duodenal Crypt Cells of Germ-free arid Con ventional Mice. Nature, p02: 884—86, 1964. 1958. 290. Lahtiharju, A. Influence of Autolytic arid Necrotic Liver Tissue on Liver Regeneration in Rat. Acta Pathol. Micro biol. Scand., Suppl. 150, pp. 1-99, 1961. 291. Lajtha, L. G. On DNA Labeling in the Study of the Dy. namics of Bone Marrow Cell Populations. In: F. Stohlman Homeostasis de los hepa en ratones C@H Mza machos. Rev. arg. cancerol., 4: 1—6, 1962. 321. MaalØe, 0. Role of Protein Synthesis in the DNA Replica tion Cycle in Bacteria. J. Cellular Comp. Physiol., 62 (Suppl. 1): 31—44,1963. 322. Maaløe, 0., and Hanawalt, P. C. Thymine the Normal DNA Replication Deficiency and Cycle. J. Mol. Biol., 3: 144—55, 1961. 323. MacDonald, R. A. “Lifespan― of Liver Cells. Autoradio tium-labelled Thymidine into Rat-Liver Deoxyribonucleic graphic Study Using Tritiated Thymidine in Normal, Cirrho Acid after Exchange Transfusion with Blood from Partially tic, and Partially Hepatectomized Rats. Arch. Internal Hepatectomized Rats. Biochim. Biophys. Acta, 51: 171—72, 1961. Med.,107:335—43, 1961. 300. Lang, N., and Sekeris, C. E. Isolation from Rat Liver Cells 324. MacDonald, Life Sci., 3: 161—67,1964. 301. Laquerrière, R., and Laumonier, R. Variations du taux R. A., and Pechet, G. S. Liver Cell Regemnera tion Due to Biliary Obstruction. Arch. Pathol., 72: 133—41, of a Nuclear RNA Fraction Having Messenger Activity. 1961. 325. MacDomnald, R. A., Rogers, A. E., and Pechet, G. S. Re d'acide désoxyribonucléique dans le foie du rat albinos aprésinjection de serum de rat hépatectomisé. Compt. rend. generation of the Liver. Relation of Regenerative Response Soc. Biol., 1962. 302. Law, L. W. Effect of Gonadectomy and Adrenalectomy on the Appearance and Incidence of Spontaneous Lymphoid Leukemia in C58 Mice. J. Nat. Cancer 303. Laws, J. 0. Tissue Regeneration Inst., 8: 157—59,1947. arid Tumour Development. Brit.J. Cancer, 13: 669—74, 1959. 304. —@ . Some Biological Aspects of Carcinogenesis in the Liver. Acta Uniio Intern. Contra Cancrum, 16: 87—90, 1960. 305. Leblond, C. P., and Sainte-Manie, G. Models for Lympho cyte and Plasmocyte Production. In: G. E. W. Wolstenn holme and M. O'Connor (eds.), Haemopoiesis. Cell Produc tionn amid its Regulation. Ciba Found. Symp, pp. 152-72. London : J.& A. Churchill, Ltd., 1960. 306. Leblond, C. P., and Walker, B. E. Renewal of Cell Popula tions. Physiol. Rev., $6: 255—76,1956. 307. Leduc, to Size of Partial 154: 286—89,1960. E. H. Mitotic Activity in the Liver of the Mouse during Inanition followed by Refeeding with Different Levels 326. . Growth Hepatectomy. anid Regeneration Lab. Invest., of the Liver. 11: 544—48, Ann. N. Y. Acad. Sci., 111: 70—84,1963. 327. Mackenzie, Latent I., arid Rous, Neoplastic P. The Experimental Changes in Tarred 73: 391—416,1941. 328. Mflkelä, 0., arid Nossal, l)isclosure of Skin. J. Exptl. Med., G. J. V. Autoradiographic Studies on the Immune Response. II. DNA Synthesis amongst Single Antibody-producing 329. Malmgren, Cells. Ibid., 115: 231—45,1962. R. A., and Mills, Liver Mitotic Stimulant of Properties of the (LMS) in Mouse Tumor W. Studies Tissue. J. Natl. Cancer Inst., @6:525-32, 1961. 330. Malyschew, B. t@berdie Rolle der Kupffer'scheni Zellemnbei aseptischer Enitzundung den Leber. Beitn. Pathol. Annat., 78: 1—15, 1927. 331. Markelova, tivity I. V. The 24-Hour Rhythm of Exocnine Epithelium of Rat of the Mitotic Pancreas. Bull. Ac Exptl. Biol. Med., 53: 74—77,1962. of Protein. Am. J. Anat., 84: 397—430,1949. 332. Marsh, J., Miller, B. E., arid Lamsorn, B. (4. Effect of Re . Cell Modulation in Liver Pathology. J. Histochem. peated Brief Stress on the Growth of Ehrlich Carcinnoma inn Cytochem., 7: 253—55,1959. the Mouse. J. Natl. Cancer Inst., 22: 971—77,1959. 309. Leduc, E. H., and Wilson, J. W. Injury to Liver Cells in Car bon Tetrachlonide Poisoning. Arch. Pathol., 65: 147—57, 333. Mazia, D. The Role of Thiol Groups inn the Structure annd Function of the Mitotic Apparatus. In: R. Benesch, R. E. 1958. Benesch, P. D. Boyer, I. M. Klotz, W. H. Middlebrook, A. 310. Leong, G. F., Grisham, J. W., Hole, B. V. , arid Albnight, M. G. Szent-Gyorgyi and D. R. Schwarz (eds.), Sulfur iii Pro L. Effect of Partial Hepatectomy on 1)NA Synthesis arid 308. Mitosis in Heterotopic Cancer Res., Partial Autografts of Rat Liver. @4:1496—1501, 1964. 311. Lesher, S., Fry, R.. J. M., and Kohn, H. I. Influence of Age on Transit Time of Cells of Mouse Intestinal Epithelium. Lab. Invest., 10: 291—300,1961. 312. Lesher, S., Walburg, H. E., arid Sacher, (1. A. Generation teimis. New York: Academic Press, Inc., 334. . The Analysis of Cell Reproduction. Sci., 90: 455—69,1960. 1959. Ann. N. Y. Acad. 335. ——. Mitosis and the Physiology of Cell l)ivisionn. In: J. Brachet and A. E. Minsky (eds.), The Cell, Vol. 3, pp. 77—412.New York: Academic Press, line., 1961. Cancer Research 1724 336. . Biochemistry of the Dividing Cell. Ann. Rev. Bio 362. Munro, . Synthetic Activities Comp. Physiol., 338. McKinney, A. Leading to Stohlman, F., and J. Brecher, tics of Cell Proliferation iii Cultures Blood. Blood, 19: 349—58,1962. 339. McLoughlin, Differentiation. Mitosis. 363. Murphy, Cellular 6@ (Suppi. 1): 123—40,1963. A., C. B. Mesenchymal Symp. Soc. Exptl. 340. McMinin, It. M. H., and Johnson, G. The Kine of Human Peripheral F. R. The Repair of Arti G. E. W. Wolstemnholme and J. Knight (eds.), The Immunologically Competent Cell, pp. 1—3. London: J. & A. Churchill, M. L. Cell Proliferation and Tumor 345. Scientific Menonn, and M. The Effects on Embryonic 346. @—. inski of Growth Effect of Cortisone of Frog. Cortisone and arid Keratimnization. on Z. Zell London: Metcalf, I). Adrenal Function in High- 370. remit Studies on the Role of Erythropoietin Ann. N. Y. Acad. Sci., 77: 677—702,1963. Mohnn, M. P. The Effects of I)ifferemit Low on Erythropoiesis. (eds.), The Biology of Hair States Nowell, P. B. Growth, C. Odartchenko, L., and Lysosomes and Rabajille, Related E. Induction arid Castrated Studies Particles. Cell In: J. Initiator of Normal Human Leukocytes. of Mitosis Cancer Res., 20: Acid Bio Acta, 38: 298—306,1960. Bond, V. P., H. Kinetics of Erythrocytic Feinendegen, L. Precursor E., and Proliferation H. W., and Block, P. 1)er Mechanismus liche Ablauf der reparativen postund intermitotischem Ges. Pathol., 376. Oordt, Euler Regeneration Zellbestand. und zeit in Geweben mit Verhandl. deut. 46: 333—340,1962. G. J. van. Male Gonada! Hormones. arid H. Heller (eds.), Comparative In: U. S. von Endocrinology, Vol. 1, pp. 154—207. London: Academic Press, Inc., 1963. 377. Osgood, Bull. mbm. sot. med. hop. Paris, 75: 893—901, 1959. 354. Molomut, N., Lazere, F., arid Smith, L. W. Effect of Audio gem ic Stress upon Methylcholannthrenie-induced Carcino genesis iii Mice. Caincer Res., 23: 1097—1101, 1963. Inc., 1961. Ann in Dog. In: L. F. Lamerton and R. J. M. Fry (eds.), Cell Proliferation, pp. 172—87. Oxford : Blackwell Scientific Pub lications, 1963. 374. Odland, Cl. F. The Fine Structure of the Interrelationship of Cells in the Human Epidermis. J. Biophys. Biochem. Cytol., 4: 529—38,1958. pp. 336—98. New darns I ‘orgarnisme (recherches expCrimenntales) . Essai d ‘applicatiorn a l'Ctude clinnico-biologique de la cirrhose ethylique Press, Phytohemagglutinsin: N., Cottier, on York: Academic Press, Inc., 1958. 353. Molimard, R. La regulation des masses paremichymateuses chez l'homme. A. 375. Oehlert, Hormonal and Malignant 462—66,1960. 372. Nygaard, 0. F., Güttes, S., annd Rusch, H. P. Nucleic Metabolism in a Slime Mold with Synchronous Mitosis. the Growth of Hair inn Rats. In: W. Montagnia arid R. A. Ellis Novikoff, chim. Biophys. Leukemia Strains of Mice. Cancer Res., 20: 1347—53,1960. 350. Mikhail, G. H. Hair Neogennesis in Rat Skits. Arch. Derma tol., 88: 713—28,1963. 351. Miranid, E. A., Prentice, T. C., arid Slaunwhite, W. H. Cur 352. H., Clark-Turn, in Cultures Perga arid of Normal on the Immune Response. I. The Kinetics of Plasma Proliferatiomn. J. Exptl. Med., 115: 209—30,1962. 373. Cortical Nu and Growth. In: W. W. Now Aspects Rats. Bull. Exptl. Biol. Med., 56: 89—92,1963. 369. Nossal, G. J. V., and Mäkelä,0. Autoradiographic 348. ——-—. The Cancer Cell. Brit. Med. Bull., 18: 187—92,1962. 349. Fundamental Tissue following Trauma in Noncastrated 371. Compounds of Deoxypentose of Glucokinase by Glucose in Rat Liver. Nature, 198: 1096— 97, 1963. 368. Nikiforova, E. N. Mitotic Activity of the Mammary Gland 168: 114—23,1962. Related of Brachet and A. E. Mirsky (eds.), The Cell, Vol. 2, pp. Hormones Growth arid Cell I)ivision in the Chick Embryo. forsch. u. mikroskop. Anat., 56: 619—24,1962. 347. Mercer, E. H. Keratin morn Press, 1961. (ed.), 423—88. New York: Academic Zoo!. Anz., Histochemistry 1964. 367. Niemeyer, Publications, Related acid of Growth, pp. 588-663. London : Elsevier Pres8, Inc., 1960. Growth. In: L. F. Lamertorn and R. J. M. Fry (eds.), Cell Pro!ifera tion, pp. 190—210. Oxford : Blackwell 1963. T. Quantitative Gann, 48: 255—61,1957. 366. Needham, A. E. Regeneration Ltd., 1963. 344. Mendelsohnn, Schiff, Ribonucleic @0l : 1194-97, 1964. cleic Acid innLiver Cell of Rat following Plasmopheresis. 45: 76—80, 1957. . Epithelial Regeneration and Wound Healing in the Oesophagus of the Cat. J. Embryo!. Exptl. Morphol., 6: 288—96,1958. 343. Medawar, P. 1). 1)efinitionn of the Immunologically Compe In: and 1965 Human Skin. J. Histochem., 8: 41—49, 1960. 364. Muskowitz, M. Growth, Differentiation and Reproduction of Aggregates of Cultured Mammalian Cells. Nature, 20$: 1233-30, 342. Cell. J., A. Messenger Nature, 365. Naora, H., arid Naora, H. Synthesis Influences on Epithelial Biol., 17: 359—88, 1963. ficial Ulcers inn the Urinary Bladder of the Cat. Brit. J. Surg., 43: 99—103, 1955. 341 . . Wound Healing iii the Gall Bladder of the Cat. Ibid., tent A. J., and Korner, Rat Liver Cytoplasm. chem., 30: 669—88,1961. 337. Vol. 25, November E. E. A Unifying Leukemias, Inst., 378. Concept Lymphomas, 18: 155—66,1957. . Regulation of Cell of the Etiology of the Nat!. Cancer arid Cancers. J. Proliferation. In: (ed), The Kinetics of Cellular Proliferation, York: Grunne & Stratton, Inc., 1959. F. Stohlman pp. 282—88.New 355. Mornod, J., Chanigeux, J.-P., arid Jacob, F. Allosteric Pro 379. Osnes, S. Influence of the Pituitary on the Erythropoietic teinns arid Cellular Conntrol Systems. J. Mol. Biol., 6: 306—29, Principle Produced in the Kidney. Brit. Med. J., i: 1153—57, 1963. 1960. 356. Momnod, J., annd Jacob, F. General Conclusions: Teleonomic 380. Paschkis, K. E. Growth-promoting Factors inn Tissues : A Mechanisms inn Cellular Metabolism, Growth, and Differen Review. Cancer Res., 18: 981—91,1958. tiatiorn. Cold Spring Harbor Symp. Quant. Biol., 26: 389— 381. Patt, H. M., arid Maloney, M. A. AnnEvaluation of Granulo 401, 1961. cytopoiesis. In: L. F. Lamerton arid R. J. M. Fry (eds.), 357. Monntagnna, W. The Structure arid Function of Skin. New Cell proliferation, pp. 157—71 . Oxford : Blackwell Scientific York: Academic Press, Inc., 1962. Publications, 1963. 358. Monntagnna, W., arid Chase, H. B. Redifferentiationn of Se 382. Pearson, A. E. G. The Effects of Reduction in Food Intake baceous Glands inn the Mouse after Total Extirpation with on Growth arid 1)ifferentiation of a Squamous-celled Car Methylcholannthrenne. Ariat. Record, 107: 83—92,1950. cinoma in Mice, with Special Reference to Chemotherapy. 359. Morntagrna, W., and Hamilton, J. B. Mitotic Activity Epidermis of the Rabbit Stimulated with Local of Testosterone Propionnate. J. Exptl. Zool., innthe Applications 110: 379—96, 1949. 360. Monntagna, W., arnd Kernyoni, P. Growth Potentials and Mi totic l)ivisiorn irs the Sebaceous Glannds of the Rabbit. Anat. Record, 103: 365—80,1949. 361. Movchann, 0. T. 24-Hour the Comical Epithelium tionn. Bull. Exptl. Bio!. Periodicity of Mitotic Activity of Rat arnd Mouse during Med., 51: 103—6, 1961. iii Starva Brit. J. Cancer, 11: 470—74,1959. 383. Peckham, B., arid Kiekhofer, W. Cellular Behavior in the Vagina! Epithelium of Estrogen-treated Rats. Am. J. Ob stet. Gynecol., 83: 1021—27,1962. 384. Peninigtori, I). G. Erythropoietin in Tissues: Features of the Renal Erythropoietin-producing System. In: P. C. Williams (ed), Hormones demic Press, 385. Perrotta, Inc., and the Kidney, pp. 201—20.London : Aca 1963. C. A. Initiation of Cell Proliferatiomn iii the Vaginal BuLL0uGH—Mitotic and Functional and Uterine Epithelia of the Mouse. Am. J. Anat., 111: 408. Raven, 195—204, 1962. 133, 1938. Bellamy, In: U. S. von Euler Endocrinology, 1963. pp. D. Adrennocortica! Relation to Experimental 14: 249—66,1954. and H. Heller 208—57. London (eds.), Hormones. Cell Cycle Comparative : Academic Press, Inc., Mouse Tissues. Nature, 199: 863, 1963. C., and Maurer, der W. Autoradiographische DNS-Verdopplungszeit Bestim verschiedener Zellarteni von Maus und Ratte bei Doppelmarkierung mit ‘H-unnd ‘4C-Thymidin. Naturwissenschaften, 23: 544—45,1962. 390. Porter, K. A., and Cooper, E. H. Recognition of Transformed Small Lymphocytes by Combined Chromosomal arid Iso Labels. Lancet, ii: 317—19, 1962. of the Liver to Injury. Arch. Pathol., 64: 278—83, 284—89; V. R., and Ono, T. Enzyme Patterns inn Rat Liver and Morris Hepatoma 5123 during Metabolic Transitions. Cold Spring Harbor Symp. Quant. Biol., 26: 355-62, 1961. 393. Prescott, D. M., and Bender, M. A. Synthesis and Behavior of Nuclear Proteins during the Cell Life Cycle. J. Cellular Comp. Physiol., 62: 175—94,1963. 394. Prescott, 1). M., and Kimball, R. F. Relation between RNA, DNA and Protein Syntheses in the Replicating Nucleus of Proc. Nat!. Acad. Sci., 47: 686—93, 1961. 395. Price, D., and Williams-Ashman, H. G. The Accessory Re productive Glannds of Mammals. In: W. C. Young (ed.), Sex arid Internal Secretions, pp. 366—448.Baltimore : Williams & Wilkins Company, 1961. 396. Puck, T. T., and Steffann, J. Life Cycle Analysis of Mam malian Cells. I. A Method for Localizing Metabolic Events within the Life Cycle, and Its Application to the Action of Colcemide and Sublethal Doses of X-Irradiation. Biophys. J.,3:379—97, 1963. 397. Pullinger, 398. 412. Approach to the Problem Bact., 57: 467—77,1945. Effect of Simple Injury Combined with Carcinogenic Chemicals on Tumour Forma Kinetics. In: L. F. Lamerton arid R. J. M. Fry (eds.) Ce!! Proliferation, pp. 18—34. Oxford: B!ackwell Scientific Publications, 1963. 400. Rabinovici, N., and Wiener, E. Liver Regeneration after Partial Hepatectomy inn Carbon Tetrachloride-induced Cirrhosis in the Rat. Gastroenterology, 40: 416—22,1961. 401. Raeka!lio, J. Histochemica! Studies of Vital and Post Skin Wounds. Ann. Med. Exptl. Biol. Fenniae, 39 (Suppi. 6): 1—105, 1961. . Histochemical Demonstration of Monoamine Oxidase in the Earliest Phase of Wound Healing. Nature, 199: 496—97, 1963. 403. Raekallio, J., and Levonen,E. Histochemical Demonstration of Transglucosylases and Glycogen in the “Lag― Phase of Wound Healing. Exptl. Mo!. Pathol., 404. . Adenosine Triphosphatase Early Wound Healing. 2: 69—73,1963. Activity Acta Pathol. of Rat Hormones A Review. Cancer Microbiol. Scand., 58: 451—56,1963. Biol. Med., 51: 110—14, 1961. 406. Rãsanen, T. Fluctuations in the Mitotic Frequency of the Glandular Stomach and Intestine of Rat under the Influence of ACTH, G!ucocorticoids, Scand., 58: 201—10,1963. H. A. Systemic Carcinomas and Their (ed.), inn Res., of the Normal Couni in the Kidney. Hormomnes amnd the Kidney, pp. 195— S. H. M. Physiology of the Uterus. New York: Epidermal Protein in Basal Cell Tumor. J. Invest. 1)ermatol., 42: 141—43, 1964. 415. Rous, P. Surmise arid Fact on the Nature of Cancer. Nature, 183: 1357—61, 1959. 416. Rous, P., arid 417. Rusch, Kidd, J. Neoplastic H. P. G. Conditional States. Extrinsic Neoplasma J. Exptl. Factors that Med., arid Sub 7$: 365—89,1941. Influence Carcimnogenesis. Physiol. Rev., 24: 177—204,1944. 418. Rusch, H. P., Johnson, R. 0., arid Kline, B. E. The Relation of Ca!oric Intake arid of Blood Sugar Mice. Cancer lies., 5: 705—12,1945. to Sarcogennesis in 419. Rusch, H. P. , arid Kline, B. E. The Effect of Exercise on the Growth of a Mouse Tumor. Ibid., 4: 116-18, 1944. 420. Rusch, H. P., Klinne, B. E., fluennce of Caloric Restrictionn Formation 421. Russo, with Ultraviolet J., arid Llanos, J. arid Baumannns, arid of I)ietary Radiation. M. E. C. A. The In Fat on Tumor Ibid., 5: 431—35,1945. Twenty-four-Hour Rhythm in the Mitotic Activity arid ins the Water and I)ry Matter Content of Regenerating Liver. Z. Zellforsch. u. mikroskop. Annat., 61: 824—28, 1964. 422. Rytomaa, T. Organ l)istribution arid Histochemical Prop erties of Eosinnophi! Gransulocytes in Rat. Acta Microbiol. Scannd., Suppl. 140, pp. 1-118, 1960. Rythmaa, T., arid Kivimniemi, K. In Vitro Pathol. Experimennts for Demonstration of Specific Feedback Factors inn Hat In: Proc. XIVth Scamnd. Conigr. Pathno!. Microbio!. Unniversitetsfor!aget, 1964. 424. Saba, G. C., Saba, P. , Carnicelli, Rhythm in the the Rate of Metabolism Emndocrinol., Adrenal Serum. Oslo: A. , amid Marescotti, Cortical of Corticosterone Secretion V. and inn innthe Rat. Acta 44: 409—12, 1963. 425. Saetren, H. A Principle of Auto-Regulation of Growth. Production of Organs Specific Mitose-Imnhibitors inn Kidney amidLiver. Exptl. Cell Res., 11: 229—32,1956. 426. . The Organ-specific Growth Inhibition of the Tubule Cells of the Rat's Kidney. Acta Chem. Scannd., 17: 889, 1963. 427. Saifran, M. Mechanisms of Adrenocortical Med. Bull., 18: 122—26,1962. Control. 428. Sallman, N. Aspects L. von, Grimes, P., and McElvain, Brit. of Mitotic Activity in Relation to Cell Proliferationn innthe Lens Epithelium. Exptl. Eye Res., 1: 449—56,1962. 429. Sanntler, J. E. Growth in the Ce!! Populatiorns of the Thyroid Gland of Rats Treated with Thiouracil. J. Enndocrinnol., 15: 151—61,1957. Skim in 405. Raitsina, S. S. Physiological Regeneration in the Mucosa in the Small Intestine of Hypophysectomized Rats. Bull. Exptl. 407. Rashkis, Reynolds, Diurnal tion in Mice. Ibid.,57: 477—81, 1945. 399. Quastler, H. The Analysis of Cell Population 402. Soc. Paul B. Hoeber, Imnc.,1949. 413. Roberts, K. B., Florey, H. W., arid Joklik, W. K. The In fluennce of Cortisorne on Cell Divisions. Quart. J. Exptl. Phys iol., $7: 239—57,1952. 414. Rothberg, S., arid Scott, E. J. vans. Absence of Normal 423. B. D. An Experimental of Trauma and Tumours. J. Pathol. . A Measure of the Stimulating Mortem Symp. 200. London: Academic Press, Inc., 1963. Threshold Eupiotes. Eggs. terpart. Ibid. , 24: 1131—36,1964. 411. Reissmannn, K. H. Erythropoietinn Formation 391. Post, J., Himes, M. B., Klein, A., arid Hoffman, J. Responses 1957. 392. Potter, Tumors: in Induced In: P. C. Williams 388. Pilgrim, C., Erb, W., and Maurer, W. I)iurnal Fluctuations in the Numbers of DNA Synthesizing Nuclei inn Various topic in Mollusc 410. Reiskin, A. B., arid Menidelsohns, M. L. A Comparison J. G., and mung C. P. Differentiation 409. Reid, E. Growth Hormone arid Adrernocortica! und Amitose. Zeit. Amnat.Entwicklunngsgeschichte, 109: 99— 389. Pilgrim, 1725 Exptl. Biol., 17: 274—84, 1963. 386. Pfuhl, W. Die mitotischeni Teilungen der Leberze!!en in Zusammenhanng mit den allgemeinien Fragenn überMitose 387. Phillips, Homeostasis Stress and Heparin. Acta Physio!. 430. Saxen, L. The Transmission of Information during Primary Embryonic Induction. In: R. J. C. Harris (ed), Biological Organization at the Cellular and Supercellular Level, pp. 211-28. London : Academic Press, Inc. , 1963. 431. Saxén,L., and Toivonen, S. Primary Embryonic Induction. London: Academic Press, Inc., 1962. 432. Sayers, G. The Adrenal Cortex arid Homeostasis. Physiol. Rev., 30: 241—320, 1950. 433. Scheving, L. E. Mitotic Activity ins the Human Epidermis. Arnat.Record, 135:7—14, 1959. 434. Scheving, L. E., and Chiakulas, J. J. Effect of Hypophysec Stress as an Inhibitor of Experi mental Tumors in Swiss Mice. Science, 116: 169—71, 1952. tomy on the 24-Hour Mitotic inn Urodele Larvae. J. Exptl. Rhythm of Corneal Epithelium Zool., 149: 39—44,1962. Cancer Research 1726 435. Schjeide, 0. A., and Wilkens, stimulated Protein Synthesis. M. Parameters Nature, of Oestrogen 201: 42-44, 1964. 436. Schneider, J. H., and Potter, V. H. Alternative Pathways of Glucose Metabolism. III. The Incorporation of Radio activity from Glucose-1-'4C into the Nucleic Acids of Re generating Rat Liver. Cancer lies., 17: 701—6,1957. 437. Schooley, J. C. Autoradiographic Observations of Plasma System. 439. Scott, J. Med. E. J. Psoriasis. van, Arch. Res., 463. Stoerk, amid Eke!, T. M. Kinetics of Hyperplasia poietini and Its Role inn the Regulation tionn. Arm. N. Y. Acad. Sci., 77: 710-24, 465. ins the Ear Epidermis Res., 25: 114—19,1961. 444. Sherwin-Weidenreich, H., Herrmanmi, of Mice. Exptl. Rothstein, Res., 19: 1150—53, 1959. @ilha,M. The Effect of Radiation on the Mitosis-stimulatinig Activity of Hepatectomized Rat Serum. Experientia, 226, 1963. 446. Silvers, W. K., amidRussell, E. S. An Experimental to Action of Genies at the Agouti 199—220,1955. Locus. J. Exptl. after Unilateral Load in its Production. 470. lIes., 19: 130: Am. J. Phys 452. Skjaeggestad, 0. Experimental Epidermal Hyperplasia in Mice. Relation to Carcinogenesis. Acta Patho!. Microbiol. Scand., Suppl. 169, pp. 1-126, 1964. 453. Slome, I). (ed). Wound Healing. London: Pergamon Press, Mitotic 455. Smythe, R. L., and Moore, R. 0. A Study of Possible Humoral in the Rat. Surgery, 44: 561— 456. Spain, 1). M., Molomut, N., and Novikoff, A. B. Cortisone arid Carcirsogenesis in Mouse Skin. I. Effect of Cortisone during Multiple Paintings with Methylcholarnthrenne. Cancer Res., 16: 138—41,1956. 457. Spector, W. G., arid Willoughby, D. A. Suppression of In creased Capillary Permeability in Injury by Monoamine Inhibitors. Nature, 186: 162—63,1960. 458. Stedmann, E., and Stedman, E. The Basic Proteins of Cell Nuclei. Phil. 95, 1951. Trans. Roy. Soc. (London), 5cr. B, 235: 565— Factors 17: 727—58,1957. inn Growth. Perspectives Biol. Hegyeli, A., Thymus and Gland McLaughlin, and Therapy : A Possible New J. Their A. Relation to Proc. Nat!. Acad. Approach. Science, . The Genesis and J. and Growth of Tumors. I. Cancer, $8: 335—50, 1940. Growth of Tumors. II. Effects of 1947. 476. . The Role of Nutrition in the Origin In: F. R. Moulton and Growth (ed.), Approaches of to Tumor Chemotherapy. Am. Assoc. Advan. Sci., Washington, D. C., 1947. 477. Tannenbaum, A., and Silverstone, H. The Genesis and Growth of Tumors. Cancer Res., 9: 162—73,1949. . Nutrition and the Genesis of Tumours. In: R. W. Raven (ed), Cancer, Vol. 1, pp. 306—34. London : Butterworth & Co., Ltd., 1957. Taylor, E. W. Relation Cycle in Mammalian of Protein Synthesis Cell Cultures. to the Division J. Cell Biol., 19: 1—18, 1963. 480. Tchoumak, M. G. Study of the Mitotic Cycle in the Corneal Epithelium of Mice Using Tritiated 481. Teir, H., and Carpen, Hypophysectomy I. Influence on the Mitotic and Stable Organs of the Rat. Scand., 47: 291—96,1959. 482. Teir, H., and Thymidine. Proc. Acad. 149: 959—62, 1963. Isotalo, A. Influence of Adrenalectomy Frequency Acta of Pathol. Cortisone I. Effect of Single Dose and Prolonged and of Some Labile Microbiol. on Mitosis. Application. Ann. Med. Exptl. Biol. Fenniae, $1: 171—80, 1953. 483. Teir, H., and Lahtiharju, A. Effect of Necrotic Liver Tissue on Regeneration in Hepatectomized Rats. Exptl. Cell Res., 24: 424—28,1961. 484. Teir, H., Rythmaa, T., Cederberg, A., and Kiviniemi, K. Studies on the Elimination of Granulocytes in the Intestinal Tract in Rat. Acta Patho!. Microbiol. Scand., 59: 311—24, 1963. 485. Terasima, Synthesis T., and Tolmach, in Synchronously L. J. Growth and Nucleic Acid Dividing Populations of HeLa Cells. Exptl. Cell Res., $0: 344—62,1963. 459. Steinberg, H., and Watson, R. H. J. Fai!ure of Growth in Disturbed Laboratory Rats. Nature, 185: 615-16, 1960. 460. Steward, F. C. The Control of Growth in Plant Cells. Sci. Am., 209: 104—13,1963. 461. Stich, H. F. Regulation of Mitotic Rate innMammalian Or Ann. N. Y. Acad. P. Caloric Restriction per se. Cancer Res., 2: 460—67,1942. 475. . Effects of Varying Caloric Intake upon Tumor In cidence annd Tumor Growth. Ann. N. Y. Acad. Sci., 49: 5—18, 1962. ganisms. A., Sci. U.S.S.R., Factors in Liver Regeneration 69, 1958. Studies of the . Cancer 474. Index, l)uration of Mitosis, Generation Time and Fraction of Dividing Cells in a Cell Population. Nature, 19$: 555—56, Oxidase Szent-Gyorgyi, 478. between Res., 473. Tannienbaum. A. The Initiation Effects of Underfeeding. Am. Nephrectomy 7: 726, 1947. P. P. Relation C. G. M. Humoral Growth, Fertility, Muscle and Cancer. Sci., 48: 1439—42,1962. 479. Dendy, Rhoads, 140: 1391—92, 1963. 602—14, 1959. C. L., and Cancer A. Constituents 451. Siskin, J. E. The Synthesis of Nucleic Acids and Proteins in the Nuclei of Tradescantia Root Tips. Exptl. Cell lIes., 16: 1961. 454. Smith, and Med., 7: 279—84, 1964. 450. Simpson, W. L. An Experimental Study of Single Trauma Cancer K., R., amid Hass, Mechanisms. Szent-Gyorgyi, 472. iol., 201: 517—22,1961. Malignancy. Dobriner, . The Control of Cell 1)ivision : A Review. II. Special Mechanisms. Ibid., 18: 1118—60, 1958. Tumors. 1). P. Hyperplasia C., corticosteromne Acetate during Early and Late Stages of Liver Carcinogenesis in Rats Fed p-Dimethy!aminoazoben zene. Acta Unio Intern. Contra Cancrum, 19: 771—73,1963. Ibid.,202: 906—7, 1964. and Role of Excretory C. Cell Produc 469. Symeonnidis,A. The Effect of Adrenalectomy and of Desoxy M. Approach Zool., Stock, J., Kennedy, General 468. Cell 447. Simmons, 1). J. I)iurnnal Periodicity in Epiphyseal Growth Cartilage. Nature, 195: 82—83,1962. 448. . Circadiann Mitotic Rhythm in Epiphyseal Cartilage. 449. Simpson, K., of Red 1959. in Liver Regeneration. Federation Proc., 21: 301, 1962. 467. Swannn, M. M. The Control of Cell Division : A Review. I. 471. F., and Im Testosterone and 74: 798—800, 1950. The Effect of Cortisone and Other Steroids on Experimental Tumors. Cancer Res., 10: 244—45,1950. J. The Effect of Cortisone and Hair Cycle on the Incidence of Chemica!ly Induced Epiderma! Tumors in Mice. Cancer 445. Sugiura, 466. Survis, Scalp Hair. J. Invest. Dermatol., 41: 269—74,1963. 441. Segal, S. Hormomnes, Aminno-Acid Transport arid Protein Synthesis. Nature, 203: 17—19,1964. 442. Selby, C. C. An Electronn Microscope Study of the Epidermis of Mammalian Skin inn Thin Sections. I. Dermo-epiderma! Junction arid Basa! Ce!! Layer. J. Biophys. Biochem. Cyto!., 1: 429—43, 1955. 443. Sherman, F. G., Quastler, H., arid Wimber, I). H. Cell Pop of Lymphosarcoma Rats by Biol. Med., 464. Stoh!mann, F. Observations on the Physiology of Erythro inn 88: 373—81,1963. 440. Scott, E. J. van, Eke!, T. M., Auerbach, B. S., and Auerbach, IL Determinants of Rate arid Kimietics of Cell Division in ulationn Kinetics H. C. Growth Retardation plants inn Pyridoxine-deficient Cortisone. Proc. Soc. Exptl. 44: 207—30, 1923. I)ermatol., 1965 462. Stich, H. F., and Florian, M. L. The Presence of a Mitosis Inhibitor in the Serum and Liver of Adult Rats. Can. J. Biochem. Physiol., 36: 855—59,1958. Cell Formation. J. Immunol., 86: 331—37, 1961. 438. Schultz, E. W., Hall, E. M., and Baker, H. V. Repair of the Liver following the Injection of Chloroform into the Portal Vol. 25, November Sci., 90: 603—9,1960. 486. Thiessen, D. D., and Nealey, V. G. Adrenocortica! Activity, Stress Response and Behavioral Reactivity of Five Inbred Mouse Strains. Endocrinology, 71: 267—70, 1962. 487. Thuringer, J. M., and Katzberg, A. A. The Effect of Age on Mitosis in the Human Epidermis. J. Invest. Dermatol., 33: 35—39, 1959. BuLL0uGH—Milotic and Functional 488. Trainin, 511. Weinbren, N. Adrenal imbalance in mouse skin carcinogenesis. N., Kaye, A. M., and Berenblum, I. Influence Mutagens on the Initiation of Skin Carcinogenesis. Pharmaco!., 1$: 263—67,1964. 490. Trott, J. R., and Gorenstein, S. L. Mitotic Rates Supply of Biochem. in the Ora! 513. and Gingival Epithe!ium of the Rat. Arch. Oral Biol., 8: 425—34, 1963. R. The Nucleic Acids during Mitotic Division. Cancer Res., 22: 881—84, 1962. of 1)evelopment. New York: Press, 1949. Biological University Specificity and Press, 1955. Growth, 515. Wennneker, A. S., and Sussman, pp. 195—206. Princeton: N. Regeneratiomi of Liver Tissue Intense Proc. Soc. Exptl. Biol. Med., 76: 683—86, 1951. Proliferation. Bull. Methodi Popoff Inst. Biol. Bulgar. Acad. Sci., 7: 193—207, 1956. in Parabiotic Rats. Protein. Content Biochem. in Pancreatic Acinar Cells in Vitro. J. Cell Biol., 20: 415—33, 1964. 518. . Tissue Innteractionns and Cytodifferentiationi. J. and l)ifferenntiation Exptl. Zoo!., 157: 139—52, 1964. metol., 6: 15—20, 1962. 496. Tumanischevili, G. D. Stimulating Action of Tissue Ex tracts on Regenerative phol., 8: 226—38,1960. Processes. F., and Halberg, Response Hepatectomy Ann. N. Y. Acad. Sci., 90: 422-29, 1960. 517. Wessels, N. K. l)NA Synthesis, Mitosis, . Etude sur les ribonucleoproteines solubles de l'epi derme par spectrophotométrie directe de l'electrophore gramme dans la region ultra-violette. Arch. Biochem. Cos Vitro Partial Stoppage sci., 12: Lab., 1: 7—13, 1962. 497. Ungar, following 516. Went, H. A. 1)ynamic Aspects of Mitotic Apparatus . Reactive Tissue Changes following Periodic of Blood Circulation. Compt. rend. Acad. bulgare 565-568, 1959. 494. . Injury-induced Changes in Nucleic Acid of the Epidermis. Bulgar. Acad. Sci. Bull. Central Henry . Specificity in Growth Control. In: E. G. Butler (ed), Histochemical Observation on the Epidermis in a State of 493. 495. Liver. . Differential Growth. In: A. K. Parpart (ed), The Chemistry and Physiology of Growth, pp. 135—86.Princeton: 514. 21: 778—82,1961. of & Co., 1939. University 491. Trotter, N. L. The Effect of Partial Hepatectomy on Sub cutaneously Transplanted Hepatomas innMice. Cancer Res., 492. Tsanev, in the Rat K. V. The Enhancement by 1)eprivationn of the Portal Blood 512. Weiss, P. Principles Holt 1727 K., arid Ghorpade, Ductal Proliferation Cancer Res., 2$: 415—419, 1963. 489. Trainin, Homeostasis J. Embryo!. F. Circadian of Mouse Adrenal to Rhythm in the in Adrenocorticotropic 499. Upton, A. C., and Furth, J. Inhibition by Cortisone of the Development of Spontaneous Proc. Leukemia Am. Assoc. Cancer and its Induction Res., 1: 57, 1953. 500. Utkin, I. A., and Kosichenko, L. P. The Role of Environ mental Conditions on Mitotic Activity. III. The Influence of Changes in the Mouse Stock Composition on Cell Division in Cornea. Bull. Exptl. Biol. Med., 41: 62—66,1956. 501. Vasama, R., and Vasama, R. On the Diurnal Cycle of Mitotic Activity in the Corneal Epithelium 230—37,1958. magai, of Mice. Acta Anat., 33: E. Adreniocortica! 1). B., Ku Function arid 520. Wetherley-Mein, G., and Cottom, D. G. Fresh Blood Tranns fusion inn Leukemia. Brit. J. Haematol., 2: 25—31,1956. 521. White, F. H. The Relationship between Unnderfeedinig arid Tumor Formation, Transplantation, and Growth inn Rats and Mice. Cancer lIes., 21: 281—90,1961. 522. White, R. G. Factors Affecting the Antibody Brit.Med. Bull.,19:207—13, 1963. 523. Williamson, M. B., and Guschlbauer, W. Changes Response. inn the Con cemntration of Ribonucleic Acid during Wound Tissue Re generation. Nature, 192: 454—55,1961. 524. . Metabolism of Nucleic Acids during Regeneration of Wound Tissue. J. Biol. Chem., 236: 1463—65,1961. 525. ———. Metabolism of Nucleic Acids during Regeneration of Wound Tissue. II. The Rate of Formation of RNA. Arch. Biochem. Biophys., 100: 245—50,1963. 526. Willis, R. A. Pathology of Tumors. St. Louis: C. V. Mosby Company, 1953. 527. Wilson, J. W., and Leduc, E. H. Mitotic Activity inn Mouse Liver following Intraperitonea! Injection of Liver, Kidney arid Egg Yolk. Ainat. Record, 97: 470—94,1947. 502. Villee, C. A. Estrogens and Uterine Enzymes. Arm. N. Y. Acad. Sci., 75: 524—34,1959. L. F., arid Ennglert, Cortisol Metabolism innOld Age. J. Clin. Endocriniol. Metab., 21: 1197—1207, 1961. Exptl. Mor Hormone. Science, 137: 1058—60,1962. 498. . In Vitro Exploration of a Circadian Rhythm in Adrenocorticotropic Activity of C Mouse Hypophysis. Ex perientia, 19: 158—60,1963. by Radiation. 519. West, C. D., Brown, H., Simons, E. L., Carter, 528. . . The Effect of Coramine on Mitotic Activity and 503. Virolainen, M. Mitotic Response in Liver Autograft after Growth in the Liver of the Mouse. Growth, 14: 31—48,1950. Partial Hepatectomy in the Rat. Exptl. Ce!! Res., 33: 588—91, 529. Wooley, G. W., and Peters, B. A. Prolongation of Life in 1964. High-Leukemia 504. Vorobyev, V. I., and Bresler, V. M. Unfractionated tions of Histones from Normal Mammalian Tissues Prepara as Agents Inhibiting Growth of Transplanted Tumours. Nature, 198: 545—47,1963. 505. Voutilainen, A. On Regional Fluctuations in the Mitotic Activity of Malignant Growths. Acta Pathol. Microbiol. AKR Mice by Cortisone. Proc. Soc. Exptl. Biol. Med., 82: 286—87,1953. 530. Wurtman, R. J., Axelrod, J., and Potter, L. T. The Disposi tion of Catecholamines in the Rat Uterus arid the Effect of Drugs and Hormones. J. Pharmacol. Exptl. Therap., 144: 150—55,1964. 531. Wurtman, R. J., Chu, E. W., and Axelrod, J. Relation be tween the Oestrous Cycle and the Binding of Catecholaminnes Scand., 26: 327—30, 1955. 506. Wallace, E. W., Wallace, H., and Mills, C. A. Influence of innthe Rat Uterus. Nature, 198: 547—48,1963. Environmental Temperature upon the Incidence and Course 532. Yaffe, D., and Feldman, M. The Effect of Actimnomycims D on of Spontaneous Tumors in C3H Mice. Cancer Res., 4: 279—81, Heart and Thigh Muscle Cells Grown in Vitro. Develop. 1944. 507. . Influence of Environmental Temperature upon the Incidence and Course of Spontaneous Tumors in Spayed Bio!., 9: 347—66,1964. 533. Yoffey, J. M. The Lymphomyeloid Complex. In: G. E. W. Wolstenholme and M. O'Connor (eds.), Haemopoiesis. Cell C3H Mice. Ibid., 5: 47—48, 1945. 508. Walsh, L. S., and Loeb, Production and Regulation, pp. 1—36. London : J. & A. Church L. Quantitative Study of Regenera tion in the Uterus of the Guinea Pig. J. Med. Res., 37: 441— 534. 70, 1918. 509. Wegener, K., Ho!lweg, S., and Maurer, W. Autoradiographi sche Bestimmung der Dauer der DNS-Verdopplung und der Generationszeit bei fetalen schaften, 50: 738—39,1963. 510. Weinbren, K. Regeneration $7: 657—68,1959. Zellen der Ratte. Naturwissen of the Liver. Gastroenterology, ill,Ltd., 1960. . The Lymphocyte. Ann. Rev. Med., 15: 125-48, 1964. 535. Young, R.. W. Cell Proliferation and Specialization during Endochondral Osteogenesis inn Young Rats. J. Cell Biol., 14: 357—70,1962. 536. Zimmerman, M., and Celozzi, E. Stimulation of Cell Division inn Normal Rat Liver by a Factor in Serum from tomized Rats. Federation Proc., 19: 139, 1960. Hepatec