Chapter 11: Heat Engines, and the Second Law of Thermodynamics
advertisement

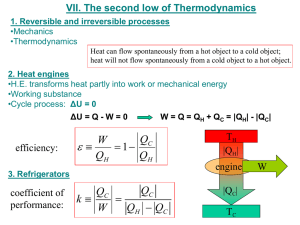
Chapter 11: Heat Engines, and the Second Law of Thermodynamics 1. The second law of thermodynamics says that the total amount of entropy, or randomness, in the universe cannot decrease. However, we see all around us objects that become more ordered - for example, the development of a biological organism, or water that freezes. The decrease in entropy, or randomness, in such cases does not violate the second law because A. there is always a greater increase in entropy somewhere else. B. energy must be added. C. energy must be removed. D. water and living things do not have entropy. Answer: A 2. Why couldn’t you use an electric motor to turn an electrical generator that in turn provides the electrical energy for the motor? A. No energy converter is 100% efficient. B. Yes, you can do this! C. Motors require AC and generators produce DC. Answer: A 3. Shown below are 4 figures that represent the heat flow in a heat engine. The thickness of the arrows represents the amount of heat flow. Which figure best represents a real heat engine? B C D A Answer: B 4. Energy added to a cyclical heat engine A. is completely converted to external work. B. is converted to increased internal energy in the engine plus external work. C. is used to generate work that is greater than the added energy. D. is converted to work and to waste heat. Answer: D 5. The primary function of any heat engine is to A. convert work into heat. B. create energy. C. convert heat into work D. destroy energy and replace it with work Answer: C 6. The work performed by a heat engine A. equals the heat energy exhausted from the engine. B. equals the heat energy entering the engine. C. equals the change in the internal energy of the engine. D. equals the net heat flow into the engine. Answer: D 7. The change in internal energy during one complete cycle of a heat engine A. equals the net heat flow into the engine. B. equals zero. C. equals the heat energy exhausted from the engine. D. equals the heat energy entering the engine. Answer: B 8. One important feature of the Carnot cycle is that it A. predicts the maximum efficiency of any engine operating between two temperatures. B. predicts the maximum work output from any real engine. C. predicts the maximum heat exhausted from any real engine. D. is the only real complete cycle which produces work. Answer: A 9. The efficiency of an engine can be defined as the A. total amount of work performed. B. ratio of work done to energy exhausted. C. ratio of work done to energy input. D. ratio of heat exhausted to heat intake. Answer: C 10. If a Carnot heat engine is run in reverse, it becomes A. a refrigerator. B. very often a failed engine. C. a very low efficiency heat engine. D. It can’t be run in reverse; to do so would violate the 1st law of Thermodynamics. Answer: A 11. A correct statement of the 2nd Law of Thermodynamics is: A. the random motion of gas molecules will be decreased if energy is added to a gas. B. there is no process that can make heat flow from a cold object to a hot object. C. no heat engine can have an efficiency greater than 30%. D. heat will not flow spontaneously from a cold object to a hot object. Answer: D 12. The word entropy is used to describe A. the total amount of work done by a system. B. the amount of disorder in a system. C. the total amount of internal energy in a system. D. the heat available from a hot gas. Answer: B 13. The overall direction of change in the universe is toward A. a state of increased mass in the universe. B. increased energy content of the universe. C. a state of greater disorder. D. a state of increased organization. Answer: C 14. An ideal heat engine is operating between high and low temperature reservoirs. Suppose that now the low temperature reservoir has its temperature lowered, but no other changes are made. This temperature change affects the engine efficiency in the following way: A. the efficiency increases. B. the efficiency is unchanged. C. the efficiency decreases. D. there is now no way to calculate the efficiency. Answer: A 15. Some power plants extract energy from the warm ocean currents. A major disadvantage to these power plants is that A. the cost of warm ocean water is quite high. B. there is no low temperature reservoir. C. the amount of heat released to the environment is unacceptable. D. the efficiency is quite low. Answer: D 16. In one cycle of a heat engine the A. net heat absorbed is zero. B. net work done is zero. C. net work done equals the heat absorbed from the high temperature heat source. D. internal energy does not change. E. heat given off is zero. Answer: D 17. A Carnot engine is A. equivalent to the gasoline internal combustion engine. B. currently in production for the new generation of imported cars. C. a theoretical engine having the highest possible efficiency for the temperatures involved. D. one that would violate the second law of thermodynamics. Answer: C 18. A Carnot engine A. consists of only isothermal processes. B. consists of only processes which do not involve heat flow. C. absorbs heat at a single high temperature and rejects heat at a single low temperature. D. consists of constant temperature and constant volume processes. E. consists of zero heat flow and constant pressure processes. Answer: C 19. A Carnot engine operating between reservoirs at 227oC and 27oC would have an efficiency of approximately: A. 0.11. B. 0.88. C. 0.60. D. 0.40. E. 0.67. Answer: D 20. A heat engine having an efficiency of 0.40 takes in 1000 J of energy from the hot reservoir in one cycle. In the same time, how much work will it perform? A. 400 J B. 500 J C. 600 J D. 800 J E. 1000 J Answer: A 21. A heat engine takes in 500 J of energy from the hot reservoir in one cycle while performing 200 J of work. The amount of heat transferred to the cold reservoir in the same time is A. 500 J. B. 400 J. C. 300 J. D. 200 J. E. zero. Answer: C 22. A heat pump is capable of delivering more energy to the home than goes into the operation of the pump itself, when conditions are favorable. Which of the following statements is correct? A. A heat pump violates the first law of thermodynamics. B. A heat pump violates the second law of thermodynamics. C. A heat pump transfers some energy from the outdoors. D. A heat pump, like the Carnot engine, is a theoretical device that is not useful in practice. E. A heat pump is more efficient when the outside temperature is colder. Answer: C 23. A refrigerator transfers heat from a colder body to a hotter body while energy is used to operate the device. This is A. a violation of the first law of thermodynamics. B. a violation of the second law of thermodynamics. C. consistent with Clausius's statement of the second law. D. only possible for a refrigerator using a Carnot cycle. Answer: C 24. Entropy is A. another term for heat. B. a quantity that is conserved in any thermal process. C. something that never decreases in any process. D. a quantity that increases as the disorder of a system increases. Answer: D 25. An inventor claims to have created a heat engine that extracts energy from the ocean and turns it all into work. Such a device is A. a violation of the first law of thermodynamics. B. a violation of Kelvin's statement of the second law of thermodynamics. C. only possible for a Carnot cycle. D. consistent with Clausius's statement of the second law of thermodynamics. Answer: B 26. Which of the following energy sources would result in the least thermal pollution when operated under favorable conditions for a given amount of energy output? A. An electric generating plant using fossil fuel. B. A nuclear electric generating plant. C. An electric generating plant using geothermal energy. D. An electric generating plant using temperature differences at different levels in the ocean. Answer: A 27. A heat engine that would take in 1000 J of heat from a reservoir at 500 K and exhaust 500 J to a reservoir at 300 K, converting the other 500 J of heat to work, is A. not thermodynamically possible. B. possible only for a Carnot cycle. C. thermodynamically possible. Answer: A 28. Which of these is characteristic of an ideal (Carnot) heat engine? A. It’s submersible. B. It’s washable. C. It’s affordable. D. It’s reversible. Answer: D 29. If, for an ideal heat engine, QH , QC, and W are, respectively, the heat absorbed, the heat rejected, and the work done per cycle, which of these is true? A. QH cannot be more than QC. B. QH cannot be more than W. C. W cannot be more than QC. D. QC cannot be more than QH. Answer: D 30. Some people claim that the theory of evolution violates the second law of thermodynamics, since that theory holds that order develops out of disorder, corresponding to a decrease in entropy. This claim A. is correct, thereby disproving the theory evolution. B. is incorrect; the second law says only that the total entropy in a closed system increases. C. if incorrect, would mean that the theory of evolution must be true. D. none of these. Answer: B 31. Suppose 150 Joules of work is done on a system and that 300 Joules of heat are delivered to the system. Which of these is predicted by the first law of thermodynamics? A. The internal energy U of the system increases by 150 J, since Q = +300 and W = +150. B. The internal energy U of the system decreases by 150 J, since Q = -300 and W = +150. C. The internal energy U of the system increases by 450 J, since Q = +300 and W = -150. D. The internal energy U of the system does not change. Answer: C 32. Which of these is impossible, by the second law of thermodynamics? A. A ship is powered by heat from the ocean, waste heat being dumped to the night sky. B. Heat taken from the refrigerator is used to cook a meal; no other energy is required. C. A heat pump delivers more heat to a house than the energy required to operate it. D. A single match carelessly discarded causes 1500 acres of woodland to burn. Answer: B 33. A process carried out at constant temperature is known as a(n) _________________ process. Answer: isothermal 34. Heat is extracted from a hot reservoir during a(n) ________________ expansion in a Carnot cycle. Answer: isothermal 35. In an ideal reversible process such as a Carnot cycle the entropy of the universe ___________________ (increases, decreases, is unchanged). Answer: is unchanged 36. A refrigerator that would transfer heat from a cold body to a hotter body without work being done would violate __________________ statement of the second law of thermodynamics. Answer: Clausius’s 37. In any process, the total ________________ in all parts of the universe increases or remains constant. Answer: entropy 38. A Carnot heat engine could have an efficiency of 100% only if the temperature of the exhaust were at ______________. Answer: 0 K or absolute zero 39. A heat engine run in reverse would be a ___________________. Answer: refrigerator 40. A heat engine that converts all the heat taken in from a single temperature source to work would be in violation of what natural law? ______________________(more than one word) Answer: Second law of thermodynamics 41. When one kilogram of ice at 0oC is converted to the same amount of water at 0oC by adding heat, the change in entropy is _______________(positive, negative, zero). Answer: positive