10. Electric Force. Coulomb`s Law
advertisement

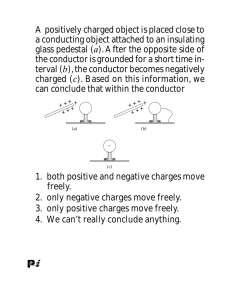
ELECTRIC FORCE (Chapter 19) Electric forces are important All interatomic and intermolecular forces are electric o i.e. forces that determine properties of materials Two kinds of charge: positive (+) and negative (-) opposite charges attract, like charges repel Net charge in an isolated system is conserved (i.e. constant) 19 Always an integral multiple of electron charge e 1.60 10 C Classify materials by electrical properties Conductor: electrons flow freely (i.e. metals) Semiconductor: “controllable” electron flow (i.e. silicon, used in electronics) Insulator: electrons stay on atoms or molecules in material Can separate positive and negative charges in materials: Can charge a conductor by INDUCTION: 1. Connection to ground allows charged rod to push like charge away o Creates charge imbalance in conductor 2. Remove ground while charged rod remains in place o Conductor remains charged 3. Charged rod removed o Conductor retains a NET CHARGE Insulators can be POLARIZED: Molecules in insulators: o may be polar (have permanent electric dipoles) o may be easily distorted to give induced electric dipole A charged object near an insulator may align molecules o Gives effective charge separation Material POLARIZED COULOMB’S LAW (1777) Experimental result: Force between two charged bodies 1 F e separated by r: r2 o Inverse square law Can measure electrical force with Torsion Balance Find torque from twist in wire Electric Force is a vector: Magnitude AND Direction Magnitude of Electric Force: Fe k e q1 q2 r2 Coulomb constant: k e 8.99 10 N m / C 9 2 2 OR ke 1 4 0 12 2 2 Permittivity of free space: 0 8.854 10 C / N m But electric force is a vector so we need to talk about direction too! Two charged particles exert force on each other: Newton’s 3rd Law Forces have equal magnitude, opposite direction Notation: F12 is the force on charge 2 caused by charge 1 Define special unit vector: r̂12 is a unit vector that points FROM 1 TO 2 Then write Coulomb’s law as: qq F12 k e 1 2 2 r̂12 r Direction of force comes out easily if used correctly q1q2 F k r̂12 e 2 Direction in Coulomb’s Law: 12 r If both charges have same sign: ( i.e. so that product q1q2 is positive) then force on 2 is in same direction as r̂12 o i.e. force on 2 is AWAY from 1 o force is repulsive If charges have opposite signs: ( i.e. so that product q1q2 is negative) then force on 2 is in opposite direction to r̂12 o i.e. force on 2 is TOWARD 1 o force is attractive By Newton’s 3 Law: F21 F12 rd (i.e. both forces attractive or both repulsive) Net force on a charge due to several other charges: VECTOR SUM of all forces on that charge due to other charges Called Principle of SUPERPOSITON Each charge exerts a force on charge 1 Resultant force is F1 F21 F31 F41 says net force on charge 1 is sum of: o force on 1 from 2, o force on 1 from 3, o force on 1 from 4 Example: Chapter 19, problem 14 Three point charges are located at corners of an equilateral triangle as shown. Calculate the resultant force on the 7.00-μC charge. Example: Chapter 19, problem 7 Two small beads having positive charges 3q and q are fixed at opposite ends of a horizontal, insulating rod, extending from the origin to the point x = d. A third small charged bead is free to slide on the rod. At what position is the third bead in equilibrium? Can it be in stable equilibrium?