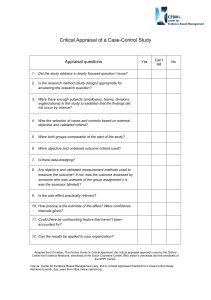
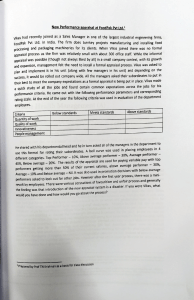
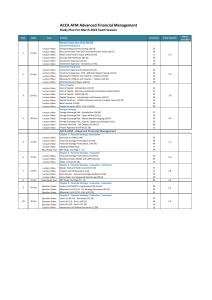
Review Proposal The planned date for review of the guidance on
advertisement

Review Proposal The planned date for review of the guidance on beta interferon and glatiramer acetate for multiple sclerosis is November 2004. This is the date at which the Institute decides whether sufficient new evidence has emerged for the Appraisal Committee to be asked to undertake a full review appraisal. The Institute has carried out a search for information relevant to this appraisal. A number of trials have reported on the use of these drugs since the publication of the guidance, and in addition the Department of Health risk sharing scheme has been established and is collecting data on those receiving the drugs under the scheme. The initial data on approximately 5000 patients in the scheme is not expected until 2007, and the Institute feels that the value in reviewing the guidance before this data is available would be limited. Consequently we propose that the decision to review the original guidance be deferred until November 2006, when the availability of data from the scheme will be assessed to ascertain the appropriateness of proceeding with a review of the guidance. A review of the guidance would be contingent on having the data and analysis from the scheme. In order to be completely confident that this is appropriate, we have asked all original consultees, to inform us of any evidence which would suggest that an earlier review would be beneficial.