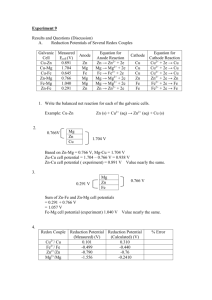
2 more examples…. Quantitative Aspects of Electrolysis
advertisement
The End of Chem1051 Classes! – Now, Review Classes 2 more examples…. • Practice lots of examples, a few each day, do not try and study all of 1051 in a couple of days, it will not work! • Read the questions! And recognise what the ‘asker’ wants! • Get help if you need it; Resource Room, Tutors/TAs, Tutorial, Me (e-mail: fkerton@mun.ca) • Gold can be plated out of a solution containing Au3+. What mass of gold (in grams) will be plated by the flow of 5.5 A of current for 25 minutes? • Silver can be plated out of a solution containing Ag+. How much time (in minutes) would it take to plate 12 g of silver using a current of 3.0 A? • Career advice - I am happy to provide this, arrange an appointment or just try and find me. Slide 50 Quantitative Aspects of Electrolysis Problem – Calculating quantities in electrolysis 1 mol e- = 96485 C PROBLEM: A technician is plating a faucet (tap) with 0.86 g of Cr from an electrolytic bath containing aqueous Cr2(SO4)3. If 12.5 min is allowed for the plating, what current is needed? PLAN: mass of Cr needed SOLUTION: Cr3+(aq) + 3e- 3mol e-/mol Cr mol of e- transferred 9.65x104C/mol 0.86g (mol Cr) (3 mol e-) C/soC omol e- omol product og product Cr(s) = 0.050 mol e- (52.00 gCr) (mol Cr) 0.050 mol e- (9.65x104 C/mol e-) = 4.8x103 C echarge (C) divide by time 4.8x103 C (min) 12.5 min (60s) Charge (C) = current (C/s) x time (s) Mass of product? divide by M mol of Cr needed Slide 49 MASS MASS(g) (g) of ofsubstance substance oxidized oxidizedor or reduced reduced M(g/mol) AMOUNT AMOUNT(MOL) (MOL) of ofsubstance substance oxidized oxidizedor or reduced reduced AMOUNT AMOUNT(MOL) (MOL) of electrons of electrons transferred transferred Faraday constant (C/mol e-) = 6.4C/s = 6.4 A balanced half-reaction current (A) CHARGE CHARGE(C) (C) time(s) Slide 48 CURRENT CURRENT(A) (A) Slide 47 Problem – Making qualitative predictions continued • Predict the half-reaction occurring at the anode and the cathode for electrolysis of each of the following: (b) Ag+(aq) + e2H2O(l) + 2e- Ag(s) E0 = -0.80V E0 = -0.42V H2(g) + 2OH-(aq) Ag+ is the cation of an inactive metal and therefore will be reduced to Ag at the cathode. Ag(s) Ag+(aq) + e- – A mixture of molten AlBr3 and MgBr2 – An aqueous solution of LiI The N in NO3- is already in its most oxidized form so water will have to be oxidized to produce O2 at the anode. O (g) + 4H+(aq) + 4e2H O(l) 2 (c) Mg2+(aq) + 2e- 2 E0 Mg(s) = -2.37V Mg is an active metal and its cation cannot be reduced in the presence of water. So as in (a) water is reduced and H2(g) is produced at the cathode. The S in SO42- is in its highest oxidation state; therefore water must be oxidized and O2(g) will be produced at the anode. Slide 46 Problem – Predicting half- and overall reactions in electrolysis Slide 45 Complications in Electrolytic Cells PROBLEM: What products form during electrolysis of aqueous solution of the following salts: (a) KBr; (b) AgNO3; (c) MgSO4? PLAN: Compare the potentials of the reacting ions with those of water, remembering to consider the 0.4 to 0.6V overvoltage. The reduction half-reaction with the less negative potential, and the oxidation halfreaction with the less positive potential will occur at their respective electrodes. SOLUTION: (a) K+(aq) + e2H2O(l) + K(s) 2e- H2(g) + 2OH-(aq) E0 = -2.93V E0 = -0.42V The overvoltage would make the water reduction -0.82 to -1.02 but the reduction of K+ is still a higher potential so H2(g) is produced at the cathode. 2Br-(aq) 2H2O(l) Br2(g) + 2eO2(g) + 4H+(aq) + 4e- • • • • Overpotential.(1) Competing reactions.(2) Non-standard states.(3) Nature of electrodes.(4) E0 = 1.07V E0 = 0.82V The overvoltage would give the water half-cell more potential than the Br-, so the Br- will be oxidized. Br2(g) forms at the anode. Slide 44 Slide 43 The tin-copper reaction as the basis of a voltaic (galvanic) and an electrolytic cell. Comparison of Voltaic and Electrolytic Cells Electrode Cell Type 'G Ecell Name Process Sign Voltaic <0 >0 Anode Oxidation - Voltaic <0 >0 Cathode Reduction + Electrolytic >0 <0 Anode Oxidation + Electrolytic >0 <0 Cathode Reduction - voltaic cell Slide 42 20-7 Electrolysis: Causing Non-spontaneous Reactions to Occur electrolytic cell Oxidation half-reaction Sn(s) Sn2+(aq) + 2eReduction half-reaction Cu2+(aq) + 2eCu(s) Overall (cell) reaction Sn(s) + Cu2+(aq) Sn2+(aq) + Cu(s) 0.0592 V [Ag+]sat’d AgI log [Ag+]0.10 M soln n Galvanic Cell: also known as voltaic Ecell = Ecell° - Eocell= 1.103 V Electrolytic Cell: Zn2+(aq) + Cu(s) ĺ Zn(s) + Cu2+(aq) Slide 41 Example 20-10 Ecell = Ecell° - Zn(s) + Cu2+(aq) ĺ Zn2+(aq) + Cu(s) Oxidation half-reaction Cu(s) Cu2+(aq) + 2eReduction half-reaction Sn2+(aq) + 2eSn(s) Overall (cell) reaction Sn(s) + Cu2+(aq) Sn2+(aq) + Cu(s) 0.417 =0 Eocell = -1.103 V x 0.0592 V log 0.100 n 0.0592 V (log x – log 0.100) 1 log x = log 0.100- 0.417 = -1 – 7.04 = -8.04 0.0592 x = 10-8.04 = 9.1 x 10-9 Batteries and Corrosion (Sections 20-5 and 20-6) omitted Slide 40 Ksp = x2 = 8.3 x 10-17 Slide 39 Example 20-10 Measurement of Ksp Using a Voltaic Cell to Determine Ksp of a Slightly Soluble Solute. Ag|Ag+(sat’d AgI)||Ag+(0.10 M)|Ag(s) With the data given for the reaction on the previous slide, calculate Ksp for AgI. Ag+(0.100 M) + e- ĺ Ag(s) Ag(s) ĺ Ag+(sat’d) + e- AgI(s) ĺ Ag+(aq) + I-(aq) Let [Ag+] in a saturated Ag+ solution be x: Ag+(0.100 M) ĺ Ag+(sat’d M) Ag+(0.100 M) ĺ Ag+(sat’d M) [Ag+]sat’d AgI 0.0592 V 0.0592 V log log Q = Ecell° Ecell = Ecell [Ag+]0.10 M soln n n Slide 38 PROBLEM: A concentration cell consists of two Ag/Ag+ half-cells. In half-cell A, electrode A dips into 0.0100M AgNO3; in half-cell B, electrode B dips into 4.0x10-4M AgNO3. What is the cell potential at 298 K? Which electrode has a positive charge? PLAN: E0cell will be zero since the half-cell potentials are equal. Ecell is calculated from the Nernst equation with half-cell A (higher [Ag+]) having Ag+ being reduced and plating out, and in halfcell B Ag(s) will be oxidized to Ag+. Slide 37 Concentration Cells Ecell = Ecell° Ecell = Ecell°Ecell = 0 - SOLUTION: Ag+(aq, 0.010M) half-cell A Ecell = E0cell - 0.0592V 1 log Ag+(aq, 4.0x10-4M) half-cell B 0.0592 V log Q n 2 H+(1 M) ĺ 2 H+(x M) 0.0592 V x2 log 12 n 0.0592 V x2 log 1 2 Ecell = - 0.0592 V log x [Ag+]dilute [Ag+]concentrated Ecell = (0.0592 V) pH Ecell = 0 V - 0.0592 log 4.0x10-2 = 0.0828V Half-cell A is the cathode and has the positive electrode. Slide 36 Slide 35 Concentration Cells Problem – Predicting spontaneous reactions for non-standard conditions Two half cells with identical electrodes but different ion concentrations. • Will the cell reaction proceed spontaneously as written for the following cell? Pt|H2 (1 atm)|H+(x M)||H+(1.0 M)|H2(1 atm)|Pt(s) Sn(s)|Sn2+(0.50 M)||Pb2+(0.0010 M)|Pb(s) 2 H+(1 M) + 2 e- ĺ H2(g, 1 atm) H2(g, 1 atm) ĺ 2 H+(x M) + 2 e2 H+(1 M) ĺ 2 H+(x M) Slide 34 PROBLEM: In a test of a new reference electrode, a chemist constructs a voltaic cell consisting of a Zn/Zn2+ half-cell and an H2/H+ half-cell under the following conditions: [Zn2+] = 0.010M [H+] = 2.5M PH = 0.30atm Example 20-8 Ecell = Ecell° - 2 Calculate Ecell at 298 K. Slide 33 0.0592 V log Q n 0.0592 V [Fe3+] Ecell = Ecell° log [Fe2+] [Ag+] n PLAN: Find E0cell and Q in order to use the Nernst equation. SOLUTION: Determining E0cell : 2H+(aq) + 2e- H2(g) E0 = 0.00V Zn2+(aq) + 2e- Zn(s) E0 = -0.76V Zn2+(aq) + 2e- E0 = +0.76V Zn(s) Ecell = E0cell - 0.0592V n log Q Q= P x [Zn2+] Ecell = 0.029 V – 0.018 V = 0.011 V H2 [H+]2 Q= (0.30)(0.010) Pt|Fe2+(0.10 M),Fe3+(0.20 M)||Ag+(1.0 M)|Ag(s) (2.5)2 Q = 4.8x10-4 Fe2+(aq) + Ag+(aq) ĺ Fe3+(aq) + Ag (s) Ecell = 0.76 - (0.0592/2)log(4.8x10-4) = 0.86V Slide 32 Slide 31 Example 20-8 20-4 Ecell as a Function of Concentration Applying the Nernst Equation for Determining Ecell. ǻG = ǻG° + RT ln Q What is the value of Ecell for the voltaic cell pictured below and diagrammed as follows? -nFEcell = -nFEcell° + RT ln Q Pt|Fe2+(0.10 M),Fe3+(0.20 M)||Ag+(1.0 M)|Ag(s) Ecell = Ecell° - RT nF ln Q Convert to log10 and calculate constants The Nernst Equation: Ecell = Ecell° - 0.0592 V log Q n Slide 30 Summary of Important Thermodynamic, Equilibrium and Electrochemistry Relationships Slide 29 Problem – Relating Keq to Eocell for a redox reaction PROBLEM: Lead can displace silver from solution: Pb(s) + 2Ag+(aq) Pb2+(aq) + 2Ag(s) As a consequence, silver is a valuable by-product in the industrial extraction of lead from its ore. Calculate K and 'G0 at 298 K for this reaction. PLAN: Break the reaction into half-reactions, find the E0 for each half-reaction and then the E0cell. SOLUTION: 2X Pb2+(aq) + 2eAg+(aq) + e- Pb(s) Ag(s) Pb(s) Pb2+(aq) + 2e+ Ag (aq) + e Ag(s) E0cell = 0.025693V ln Keq n n x E0cell (2)(0.93V) lnK = = 0.025693V 0.025693V Slide 28 E0 = -0.13V E0 = 0.80V E0 = 0.13V E0 = 0.80V E0cell = 0.93V 'G0 = -nFE0cell = -(2)(96.5kJ/mol*V)(0.93V) K = 2.75x1031 'G0 = -1.8x102kJ Slide 27 Relationship Between E°cell and Keq Relative Reactivities (Activities) of Metals 1. Metals that can displace H from acid ǻG° = -RT ln Keq = -nFE°cell E°cell = RT nF 2. Metals that cannot displace H from acid ln Keq 3. Metals that can displace H from water 4. Metals that can displace other metals from solution Slide 26 The Behavior of Metals Toward Acids Problem – Making qualitative predictions • Peroxodisulfate salts such as Na2S2O8 are good M(s) ĺ M2+(aq) + 2 e- E° = -E°M2+/M 2 H+(aq) + 2 e- ĺ H2(g) 2 H+(aq) + M(s) ĺ H2(g) + Slide 25 oxidizing agents used in bleaching. Dichromates E°H+/H2 = 0 V such as K2Cr2O7 have been used as laboratory M2+(aq) oxidizing agents. Which is the better oxidizing E°cell = E°H+/H2 - E°M2+/M = 0 -E°M2+/M = -E°M2+/M agent in acidic solution under standard conditions, Cr2O72- or S2O82-? When E°M2+/M < 0, E°cell > 0. Therefore ǻG° < 0. Metals with negative reduction potentials react with acids All metals below hydrogen in table of E o should displace H2(g) from acidic solutions (Pb through Li) Slide 24 Slide 23 Problem – Applying the criterion for spontaneous change in a redox reaction Spontaneous Change PROBLEM: (a) Combine the following three half-reactions into three balanced equations (A, B, and C) for spontaneous reactions, and calculate E0cell for each. (b) Rank the relative strengths of the oxidizing and reducing agents: (1) NO3-(aq) + 4H+(aq) + 3e(2) N2(g) + 5H+(aq) + 4e- N2H5+(aq) (3) MnO2(s) +4H+(aq) + 2ePLAN: NO(g) + 2H2O(l) E0 = 0.96V E0 = -0.23V Mn2+(aq) + 2H2O(l) E0 = 1.23V Put the equations together in varying combinations so as to produce (+) E0cell for the combination. Since the reactions are written as oxidation, reverse the sign of – Reaction proceeds spontaneously as written. • E°cell = 0 – Reaction is at equilibrium. • E°cell < 0, (Ecell -ve) reductions, remember that as you reverse one reaction for an E0. • ǻG < 0 for spontaneous change. • Therefore: • E°cell > 0, (Ecell +ve) Balance the number of electrons – Reaction proceeds in the reverse direction spontaneously. gained and lost without changing the E0. In ranking the strengths, compare the combinations in terms of E0cell. Slide 22 Combining Half Reactions Fe3+(aq) + 3e- ĺ Fe(s) Fe2+(aq) + 2e- ĺ Fe(s) Fe3+(aq) + 3e- ĺ Fe(s) 20-3 Ecell, ǻG, and Keq • Cells do electrical work. E°Fe3+/Fe = ? – Moving electric charge. E°Fe2+/Fe = -0.440 V ǻG° = +0.880 J Fe3+(aq) + 3e- ĺ Fe2+(aq) Slide 21 Zelec = nFE • Faraday constant, F = 96,485 C mol-1 E°Fe3+/Fe2+ = 0.771 V ǻG° = -0.771 J E°Fe3+/Fe = +0.331 V ǻG° = +0.109 V ǻG = -nFE ǻG° = -nFE° ǻG° = +0.109 V = -nFE° E°Fe3+/Fe = +0.109 V /(-3F) = -0.0363 V Slide 20 Slide 19 Problem – Determining Eo from an Eocell measurement PROBLEM: A voltaic cell houses the reaction between aqueous bromine and zinc metal: Br2(aq) + Zn(s) Zn2+(aq) + 2Br-(aq) E0cell = 1.83V Calculate E0bromine given E0zinc = -0.76V anode: Zn(s) Zn2+(aq) + 2e- E0Zn as Zn2+(aq) + 2e- A new battery system currently under study for possible use in electric vehicles is the zinc-chlorine battery. The overall reaction producing electricity in this cell is Zn(s) + Cl2(g) o ZnCl2(aq). What is Eocell of this voltaic cell? PLAN: The reaction is spontaneous as written since the E0cell is (+). Zinc is being oxidized and is the anode. Therefore the E0bromine can be found using E0cell = E0cathode - E0anode. SOLUTION: Problem – Calculating Eocell for a reaction E = +0.76 Zn(s) is -0.76V E0cell = E0cathode - E0anode = 1.83 = E0bromine - (-0.76) E0bromine = 1.83 - 0.76 = +1.07 V Slide 18 Standard Reduction Potentials Slide 17 Measuring Standard Reduction Potential anode Slide 16 cathode cathode anode Slide 15 Standard Cell Potential Reduction Couples Pt|H2(g, 1 bar)|H+(a = 1) || Cu2+(1 M)|Cu(s) E°cell = 0.340 V Cu2+(1M) + 2 e- ĺ Cu(s) E°Cu2+/Cu = ? E°cell = E°right - E°left Pt|H2(g, 1 bar)|H+(a = 1) || Cu2+(1 M)|Cu(s) E°cell = 0.340 V anode cathode E°cell = E°Cu2+/Cu - E°H+/H2 0.340 V = E°Cu2+/Cu - 0 V Standard cell potential: the potential difference of a cell formed from two standard electrodes. E°Cu2+/Cu = +0.340 V H2(g, 1 atm) + Cu2+(1 M) ĺ H+(1 M) + Cu(s) E°cell = 0.340 V E°cell = E°(right)cathode - E°(left)anode Slide 14 Standard Electrode Potential, E° Slide 13 Standard Hydrogen Electrode 2 H+(a = 1) + 2 e- l H2(g, 1 bar) • E° defined by international agreement. • The tendency for a reduction process to occur at an electrode. – All ionic species present at a=1 (approximately 1 M). – All gases are at 1 bar (approximately 1 atm). – Where no metallic substance is indicated, the potential is established on an inert metallic electrode (ex. Pt). Slide 12 E° = 0 V Pt|H2(g, 1 bar)|H+(a = 1) Slide 11 Problem - Representing a Redox Reaction by Means of a Cell Diagram 20-2 Standard Electrode Potentials • Cell voltages, the potential differences between electrodes, are among the most precise scientific measurements. • The potential of an individual electrode is difficult to establish. • Arbitrary zero is chosen. PROBLEM: Diagram, show balanced equations, and write the notation for a voltaic cell that consists of one half-cell with a Cr bar in a Cr(NO3)3 solution, another half-cell with an Ag bar in an AgNO3 solution, and a KNO3 salt bridge. Measurement indicates that the Cr electrode is negative relative to the Ag electrode. PLAN: Identify the oxidation and reduction reactions and write each halfreaction. Associate the (-)(Cr) pole with the anode (oxidation) and the (+) pole with the cathode (reduction). SOLUTION: e- Oxidation half-reaction Cr(s) Cr3+(aq) + 3e- The Standard Hydrogen Electrode (SHE) Reduction half-reaction Ag+(aq) + eAg(s) Voltmeter salt bridge Cr K+ Ag NO3Cr3+ Ag+ Overall (cell) reaction Cr3+(aq) + 3Ag(s) Cr(s) + 3Ag+(aq) Cr(s) | Cr3+(aq) || Ag+(aq) | Ag(s) Slide 10 Slide 9 Terminology A voltaic (galvanic) cell based on the zinc-copper reaction. • Galvanic cells. – Produce electricity as a result of spontaneous reactions, e.g. Cells on slides 4 & 6 • Electrolytic cells. – Non-spontaneous chemical change driven by electricity. • Couple, M|Mn+ Oxidation half-reaction Zn(s) Zn2+(aq) + 2e- Reduction half-reaction Cu(s) Cu2+(aq) + 2e- – A pair of species related by a change in number of e-. Overall (cell) reaction Zn2+(aq) + Cu(s) Zn(s) + Cu2+(aq) Slide 8 Slide 7 Terminology Terminology • Electromotive force, Ecell. – The cell voltage or cell potential. • Cell diagram. – Shows the components of the cell in a symbolic way. – Anode (where oxidation occurs) on the left. – Cathode (where reduction occurs) on the right. • Boundary between phases shown by |. • Boundary between half cells (usually a salt bridge) shown by ||. Ecell = 1.103 V Slide 6 An Electrochemical Cell Slide 5 An Electrochemical Half-Cell Anode Cathode Slide 4 Slide 3 Electrode Potentials and Their Measurement General Chemistry Principles and Modern Applications Petrucci • Harwood • Herring • Madura 9th Edition Cu(s) + 2Ag+(aq) Cu(s) + Zn2+(aq) Chapter 20: Electrochemistry Cu2+(aq) + 2 Ag(s) No reaction Fran Kerton Memorial University of Newfoundland, St. John’s, Canada A1B 3X7 Slide 2