SRA 2014 Annual Meeting - San Diego, CA CRA Body of
advertisement
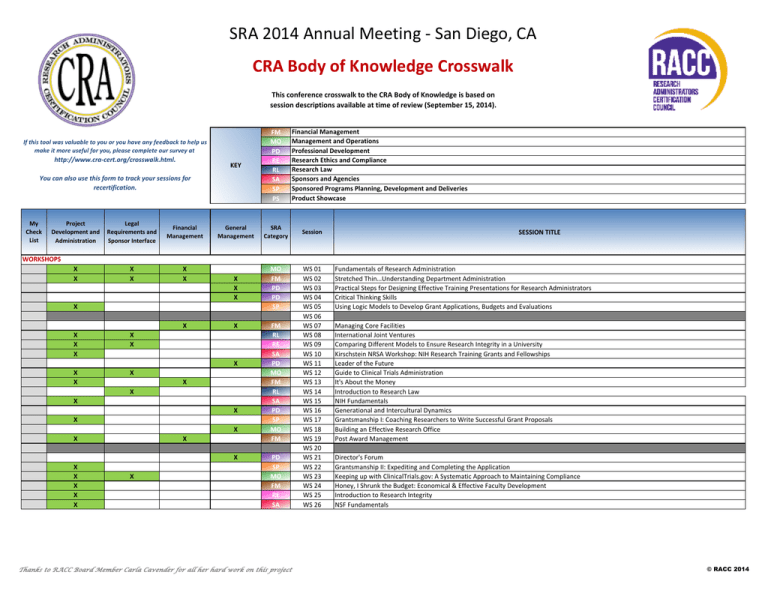
SRA 2014 Annual Meeting - San Diego, CA CRA Body of Knowledge Crosswalk This conference crosswalk to the CRA Body of Knowledge is based on session descriptions available at time of review (September 15, 2014). KEY FM MO PD RE RL SA SP PS General Management SRA Category X X X MO FM PD PD SP If this tool was valuable to you or you have any feedback to help us make it more useful for you, please complete our survey at http://www.cra-cert.org/crosswalk.html. You can also use this form to track your sessions for recertification. My Check List Project Development and Administration Legal Requirements and Sponsor Interface Financial Management X X X X Financial Management Management and Operations Professional Development Research Ethics and Compliance Research Law Sponsors and Agencies Sponsored Programs Planning, Development and Deliveries Product Showcase Session SESSION TITLE WORKSHOPS X X X X X X X X X X X X X X X X X X X X X X X X X X X X X FM RL RE SA PD MO FM RL SA PD SP MO FM PD SP MO FM RE SA Thanks to RACC Board Member Carla Cavender for all her hard work on this project WS 01 WS 02 WS 03 WS 04 WS 05 WS 06 WS 07 WS 08 WS 09 WS 10 WS 11 WS 12 WS 13 WS 14 WS 15 WS 16 WS 17 WS 18 WS 19 WS 20 WS 21 WS 22 WS 23 WS 24 WS 25 WS 26 Fundamentals of Research Administration Stretched Thin…Understanding Department Administration Practical Steps for Designing Effective Training Presentations for Research Administrators Critical Thinking Skills Using Logic Models to Develop Grant Applications, Budgets and Evaluations Managing Core Facilities International Joint Ventures Comparing Different Models to Ensure Research Integrity in a University Kirschstein NRSA Workshop: NIH Research Training Grants and Fellowships Leader of the Future Guide to Clinical Trials Administration It's About the Money Introduction to Research Law NIH Fundamentals Generational and Intercultural Dynamics Grantsmanship I: Coaching Researchers to Write Successful Grant Proposals Building an Effective Research Office Post Award Management Director's Forum Grantsmanship II: Expediting and Completing the Application Keeping up with ClinicalTrials.gov: A Systematic Approach to Maintaining Compliance Honey, I Shrunk the Budget: Economical & Effective Faculty Development Introduction to Research Integrity NSF Fundamentals © RACC 2014 SRA 2014 Annual Meeting - San Diego, CA CRA Body of Knowledge Crosswalk This conference crosswalk to the CRA Body of Knowledge is based on session descriptions available at time of review (September 15, 2014). http://www.cra-cert.org/crosswalk.html. KEY General Management SRA Category Session PD PD SP SP MO MO FM FM RL RL RE RE SA SA PS M 01 M 02 M 03 M 04 M 05 M 06 M 07 M 08 M 09 M 10 M 11 M 12 M 13 M 14 x100 Bridging the Gap: Central Administration and Academic Departments Working Together Managing Your Professional Brand Writing Goals, Hypotheses and Specific Aims Leveraging Data to Tell a Story How to Host a Successful Federal Site Visit The Ins and Outs of PI Transfers: A Departmental Perspective Strategies for Success: Assisting Principal Investigators in Funding Projects Between Awards Recent OIG Audit Findings in Brief! Immigration Options for Hiring Foreign Nationals at Research Related Organizations How to Think Like a Lawyer Creating an Institutional Environment that Supports Research Integrity International Developments in RCR and Monitoring Requirements European Funding Opportunities for US and International Researchers The Federal Demonstration Partnership (FDP): What Are They Up To? RosterTech Learning & Data Management System PD PD SP SP MO MO FM FM RL RL RE RE SA SA PS M 15 M 16 M 17 M 18 M 19 M 20 M 21 M 22 M 23 M 24 M 25 M 26 M 27 M 28 x102 Engage, Manage and Resolve: Striving for Common Ground to Reduce Conflict Strategies for Building Relationships with Faculty Stepping Out of the Silo: Success Through Breaking Organizational Boundaries Rethinking the Role of Pre-Award Sponsored Programs Administrators Research Institutes and Universities: Are We All Apples? Using Philanthropy to Support Research Pricing, Contract Research and Services Nuts and Bolts of A-133 Audit: Process and Groundwork Intellectual Property in Clinical Trials: Rogaine and Brown M&M's Navigating International Projects How to Prepare and Respond to an FDA/OHRP Inspection Ethical Challenges Facing Research Administrators in Our Day to Day Jobs! NIH Grants Administration: Who's Who and What They Do Research Support Services in an International Context: What is Required? InfoEd Electronic Records Transparency & Compliance: Today and Tomorrow You can also use this form to track your sessions for recertification. My Check List Project Development and Administration Legal Requirements and Sponsor Interface Financial Management MONDAY CONCURRENT SESSIONS 9:30 AM - 10:45 AM X X X X X X X X X X X X X X X X X X X X MONDAY CONCURRENT SESSIONS 11:15 AM - 12:30 PM X X X X X X X X X X X X X X X X X Financial Management Management and Operations Professional Development Research Ethics and Compliance Research Law Sponsors and Agencies Sponsored Programs Planning, Development and Deliveries Product Showcase FM MO PD RE RL SA SP PS If this tool was valuable to you or you have any feedback to help us make it more useful for you, please complete our survey at X X Thanks to RACC Board Member Carla Cavender for all her hard work on this project SESSION TITLE © RACC 2014 SRA 2014 Annual Meeting - San Diego, CA CRA Body of Knowledge Crosswalk This conference crosswalk to the CRA Body of Knowledge is based on session descriptions available at time of review (September 15, 2014). KEY FM MO PD RE RL SA SP PS General Management SRA Category X PD SP SP MO MO FM RL RL RE RE SA PS If this tool was valuable to you or you have any feedback to help us make it more useful for you, please complete our survey at http://www.cra-cert.org/crosswalk.html. You can also use this form to track your sessions for recertification. My Check List Project Development and Administration Legal Requirements and Sponsor Interface Financial Management Financial Management Management and Operations Professional Development Research Ethics and Compliance Research Law Sponsors and Agencies Sponsored Programs Planning, Development and Deliveries Product Showcase Session SESSION TITLE MONDAY CONCURRENT SESSIONS 2:00 PM - 3:15 PM X X X X X X X X X X X X X MONDAY CONCURRENT SESSIONS 3:45 PM - 5:00 PM X X X X X X X X X X X X X X X X PD PD SP SP MO MO FM FM RL RL RE SA SA PS Thanks to RACC Board Member Carla Cavender for all her hard work on this project M 29 M 30 M 31 M 32 M 33 M 34 M 35 M 36 M 37 M 38 M 39 M40 x104 M 41 M 42 M 43 M 44 M 45 M 46 M 47 M 48 M 49 M 50 M 51 M 52 M 53 M 54 x106 Leadership Succession Planning: Developing a Strategy Barriers and Facilitators of Broader Impacts Letters of Intent, White Papers, Preproposals and Other Short Papers Community Based Research in a Developing Country: Tips! Six Common Research Strategies and Their Effective Implementation OMB Uniform Guidance How to Handle HIPAA Breaches: The IRB and Compliance Working Together Dealing with the False Claims Act at Research Institutions Collaborative Approaches to Research Misconduct: Kick it to the RIO Building Responsible Conduct of Research Training Programs at PUIs Managing NIH Awards with Foreign Components I Measuring Your Researchers' Output with PlumX My Career in Research Administration - A Personal Review The Leadership Buffet More Paper Out the Door: Ten Inexpensive Ways to Stimulate Proposal Development How to Survive Without Departmental Administrators PUI Style Enhancing Research Administration and Management Through Partnerships Crisis Communication for the Research Administrator A Frank Discussion on Service Centers Fiscal Fitness Guide: Inspect What You Expect Negotiating Strategies and Tactics with Industry A Practical Approach to Export Control Compliance AaRPP - Authorship and Responsible Publication Practices Managing NIH Awards with Foreign Components II Proposal Development: The USAMRMC Broad Agency Announcement Evisions: Increasing Customer Service and Efficiency with the Right ERA Tool © RACC 2014 SRA 2014 Annual Meeting - San Diego, CA CRA Body of Knowledge Crosswalk This conference crosswalk to the CRA Body of Knowledge is based on session descriptions available at time of review (September 15, 2014). http://www.cra-cert.org/crosswalk.html. KEY General Management SRA Category Session X X PD PD SP SP MO MO FM FM RL RL RE RE SA SA T 01 T 02 T 03 T 04 T 05 T 06 T 07 T 08 T 09 T 10 T 11 T 12 T 13 T 14 Pragmatic Discussion of Leadership in the Current Declining Funding Environment Young Research Administrators and the Quest for Respect Delivering Research Impact in Humanities and Social Science Standard Operating Procedures and Training Programs: Central and Departmental Academic Medical Centers & Clinical Trials: Can They Peacefully Coexist? Research Administration as a Campus Shared Service? Lessons Learned Today's F&A Rate Proposal Process: What Central/Departmental Administrators Should Know Best Practices for Cost Sharing Negotiating Confidentiality Agreements - The Basics Risky Business: Social Media in Research Understanding the Various Levels of IRB Review Biosafety: Understanding and Maximizing the Institutional Biosafety Committee National Institutes of Health (NIH) Update Successful University-Industry Collaborations PD PD SP SP MO MO FM FM RL RL RE RE SA SA PS T 15 T 16 T 17 T 18 T 19 T 20 T 21 T 22 T 23 T 24 T 25 T 26 T 27 T 28 x202 Customer Service Skills for Research Administrators Training University Research Administrators: VCU Case Study Using Your Young Academics to Grow Your Research Base Critiquing Proposals When You're Not the Subject Matter Expert Setting Up Research Support Office in a Resource Limited Environment The Role of Procurement in the Administration of Research Decreasing Turnaround Times in the Budgeting and Contracting Process Lifecycle Management Strategies for Monitoring Subrecipients - The "New Uniform Guidance" and…BEYOND White Coat Crime Contract Law 101: Contracts, Cooperative Agreements, Sub-awards, Sub-contracts, Memos of Understanding Ethical Dimensions of Mobile Health Devices in Research Clinical Research Compliance in the Field: What Really Works? National Science Foundation (NSF) Update Working with DARPA: A View From the Department Level Altmetrics: A Practical Introduction You can also use this form to track your sessions for recertification. My Check List Project Development and Administration Legal Requirements and Sponsor Interface Financial Management Financial Management Management and Operations Professional Development Research Ethics and Compliance Research Law Sponsors and Agencies Sponsored Programs Planning, Development and Deliveries Product Showcase FM MO PD RE RL SA SP PS If this tool was valuable to you or you have any feedback to help us make it more useful for you, please complete our survey at SESSION TITLE TUESDAY CONCURRENT SESSIONS 9:30 AM - 10:45 AM X X X X X X X X X X X X X X X X X X TUESDAY CONCURRENT SESSIONS 11:15 AM - 12:30 PM X X X X X X X X X X X X X X X X X X X X X X Thanks to RACC Board Member Carla Cavender for all her hard work on this project © RACC 2014 SRA 2014 Annual Meeting - San Diego, CA CRA Body of Knowledge Crosswalk This conference crosswalk to the CRA Body of Knowledge is based on session descriptions available at time of review (September 15, 2014). http://www.cra-cert.org/crosswalk.html. KEY General Management SRA Category Session X X X PD PD SP SP MO MO FM FM T 29 T 30 T 31 T 32 T 33 T 34 T 35 T 36 RL T 37 RL RE RE SA SA PS T 38 T 39 T 40 T 41 T 42 x204 PD PD SP SP MO MO FM FM RL RL RE RE SA T 43 T 44 T 45 T 46 T 47 T 48 T 49 T 50 T 51 T 52 T 53 T 54 T 55 T 56 x206 You can also use this form to track your sessions for recertification. My Check List Project Development and Administration Legal Requirements and Sponsor Interface Financial Management Financial Management Management and Operations Professional Development Research Ethics and Compliance Research Law Sponsors and Agencies Sponsored Programs Planning, Development and Deliveries Product Showcase FM MO PD RE RL SA SP PS If this tool was valuable to you or you have any feedback to help us make it more useful for you, please complete our survey at SESSION TITLE TUESDAY CONCURRENT SESSIONS 2:00 PM - 3:15 PM X X X X X X X X X X X X X X X X X X X TUESDAY CONCURRENT SESSIONS 3:45 PM - 5:00 PM X X X X X X X X X X X X X X X X X X X X PS Thanks to RACC Board Member Carla Cavender for all her hard work on this project 86,400 Seconds: Tools for Time Management You Don’t Have to Do It Alone… Evaluating Research Administration: Metrics and Customer Satisfaction Surveys Building a Basic Grant Budget Open Access and Research Data Management Successful Faculty Recruitment Budget Negotiations and Financial Basics for Industry-Sponsored Research Compensation Practice and Compliance Students, Interns, Volunteers and Visitors as Researchers/Issues with Hiring and Collaborating with Non-U.S. Nationals Abroad (Export Controls and IP Issues) What is Captive Insurance and Why Should it be Considered? Confronting Challenges in Compliance: Changing Policies, Procedures and Structure/Organization Collaborative Science and the Role of the Researcher in Society Using Inspector General Work Plans to Drive Compliance Program Activities An Unexpected Journey: NIH Grant Transfers Improving Sponsored Research Information Management with Sophia Public Engagement in a Knowledge Translation Event Using Social Media Strategies for Career Advancement: Three Case Studies Broadening Your Funding Globally: Embassies, Their Governments, and IHE Partnerships Strategies for Managing Successful Partnerships With Industry Contract Timelines - Going LEAN Organizing (and Re-Organizing) University Research Offices Cost Transfers Building a Better Budget: How Budgeting Improves Clinical Trials US Patent Law Has Changes, Have Your Procedures? Indemnification and Warranties Adult Learning Styles for Electronic Research Compliance Training Challenges for Universities When Faculty Conduct Research in Other Locations Collaborating With Department of Energy Laboratories eRegulatory: Standardizing Processes and Best Practices in Collaboration with Leading Research Organizations © RACC 2014 SRA 2014 Annual Meeting - San Diego, CA CRA Body of Knowledge Crosswalk This conference crosswalk to the CRA Body of Knowledge is based on session descriptions available at time of review (September 15, 2014). http://www.cra-cert.org/crosswalk.html. KEY General Management SRA Category Session X X PD PD SP SP MO MO FM FM RL RL RE RE SA SA PS W 01 W 02 W 03 W 04 W 05 W 06 W 07 W 08 W 09 W 10 W 11 W 12 W 13 W 14 x300 Career Evolutions: From Managing Grants to Managing Grant Managers Stress Management and the Research Administrator Top Ten Tips for Successfully Working with Faculty Researchers eRA Overview/Submission Technologies We Are All in This Together Case Study: How to Conduct an In-Depth Evaluation of Research Administration Effectiveness Grant Accounting Closeouts and Other Financial Compliance Hot Topics F&A Cost Rates and the Uniform Guidance: A Non-Profit Perspective Update on the Sunshine Act Small Technology Transfer Offices and Integration with University Research Administration I Know It's Not Human Subject Research, But… Data Overload! Does Your PHI/IP Leave When a PI Does? Federal R&D Update Understanding the Agency Review Process Sponsored Program Management in the Cloud with rSmart PD PD SP SP MO MO FM W 15 W 16 W 17 W 18 W 19 W 20 W 21 W 22 W 23 W 24 W 25 W 26 W 27 W 28 Find Your Happy Place: Achieving Work-Life Balance Office Politics: Taming the 800 Pound Gorilla in the Room The Good, The Practical, and the Possible New Ways to Assess Research Impact Here We Go AGAIN! Selecting and Implementing an eRA System A Model of Integrated Research Support Managing, Tracking, Verifying and Certifying Effort You can also use this form to track your sessions for recertification. My Check List Project Development and Administration Legal Requirements and Sponsor Interface Financial Management Financial Management Management and Operations Professional Development Research Ethics and Compliance Research Law Sponsors and Agencies Sponsored Programs Planning, Development and Deliveries Product Showcase FM MO PD RE RL SA SP PS If this tool was valuable to you or you have any feedback to help us make it more useful for you, please complete our survey at SESSION TITLE WEDNESDAY CONCURRENT SESSIONS 9:30 AM - 10:45 AM X X X X X X X X X X X X X X WEDNESDAY CONCURRENT SESSIONS 11:15 AM - 12:30 PM X X X X X X X X X X X X X X RL RL RE RE SA SA Thanks to RACC Board Member Carla Cavender for all her hard work on this project My Toaster Has a MAC Address Negotiating Clinical Study Agreements with Industry - The Key Issues Is It Worth the Risk: Regulatory Non-Compliance and Institutional Risk Using "The Lab" to Educate About RCR Collaborative Approaches to Industry Engagement Global Funding: The Dawn of a New Era? © RACC 2014


