Midterm 1 Quiz W13
advertisement
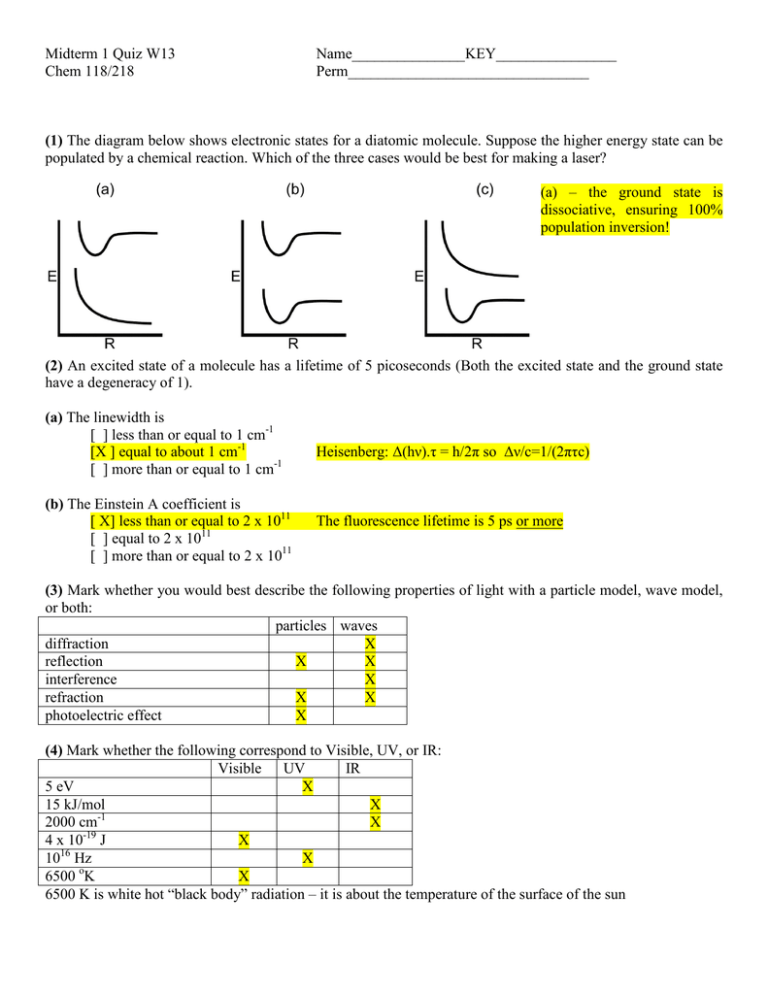
Midterm 1 Quiz W13 Chem 118/218 Name_______________KEY________________ Perm________________________________ (1) The diagram below shows electronic states for a diatomic molecule. Suppose the higher energy state can be populated by a chemical reaction. Which of the three cases would be best for making a laser? (a) – the ground state is dissociative, ensuring 100% population inversion! (2) An excited state of a molecule has a lifetime of 5 picoseconds (Both the excited state and the ground state have a degeneracy of 1). (a) The linewidth is [ ] less than or equal to 1 cm-1 [X ] equal to about 1 cm-1 [ ] more than or equal to 1 cm-1 (b) The Einstein A coefficient is [ X] less than or equal to 2 x 1011 [ ] equal to 2 x 1011 [ ] more than or equal to 2 x 1011 Heisenberg: Δ(hν).τ = h/2π so Δν/c=1/(2πτc) The fluorescence lifetime is 5 ps or more (3) Mark whether you would best describe the following properties of light with a particle model, wave model, or both: particles waves diffraction X reflection X X interference X refraction X X photoelectric effect X (4) Mark whether the following correspond to Visible, UV, or IR: Visible UV IR 5 eV X 15 kJ/mol X 2000 cm-1 X -19 4 x 10 J X 16 10 Hz X 6500 oK X 6500 K is white hot “black body” radiation – it is about the temperature of the surface of the sun (5) Circle True or False: The sum of all primary quantum yields is always less than 1: T F Each quantum of absorbed radiation excites 1 particle: T F At the isosbestic point the absorption cross sections, σ, for all absorbing components of the reaction are equal T F Population inversion can never occur in a three level system T F g to u transitions are usually forbidden T F In atoms Δl=-1 transitions are generally allowed T F always =1 (6) A sample in a 1 cm cuvette has a transmittance of 10% when its concentration is 10-3 M. (a) What is the molar extinction coefficient (include units)? Beer’s law: A=-log(0.1)=1=ε.1.10-3 so ε=1000 L.mol-1.cm-1 (b) This is measured at a wavelength at the maximum of an absorption line with a 230 cm-1 half width. The oscillator strength is roughly: [ ] 10-9 [X] 10-3 f=4.32 x 109 x (1000 x 230) [ ] 0.1 [ ] 0.5 [ ] 1 What are the units for the oscillator strength? dimensionless (it is a ratio) (7) List at least 4 effects that can contribute to linewidth and mark whether its contribution to the lineshape will be Gaussian (G) or Lorentzian (L): (a) ____Doppler___________________________ G L (b)____Heisenberg/natural___________________ G L (c)_____pressure__________________________ G L (d)______power___________________________ G L (8) List at least five possible processes that can occur following absorption of a photon: (a) _____fluorescence – phosphorescence - luminescence___ (b)______quenching________________________________ (c)______dissociation________________________________ (d)______isomerization_ internal conversion ______________ (e)______energy transfer (inter or intra molecular)__ - intersystem crossing_ (9) Given the diagram on the left, which products could you generate by photoexcitation of O2 from the v=0 level in the electronic ground state (mark all that apply): [ [ [ [ ] O2, electronically excited in low vibrational levels ] O2, electronically excited in high vibrational levels ] O atoms, triplet and singlet in about equal amounts ] O atoms, with about 3 times as many in triplet state than in singlet state The first two excited states shown are spin forbidden. Only the third state is allowed and a vertical transition excites above the dissociation limit, thus producing (3P)+O(1D). The first choice is FC allowed into the bottom of the first two excited states but very weak because of the spin change.


