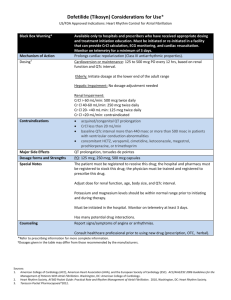
Critical Values/Critical Results List
advertisement
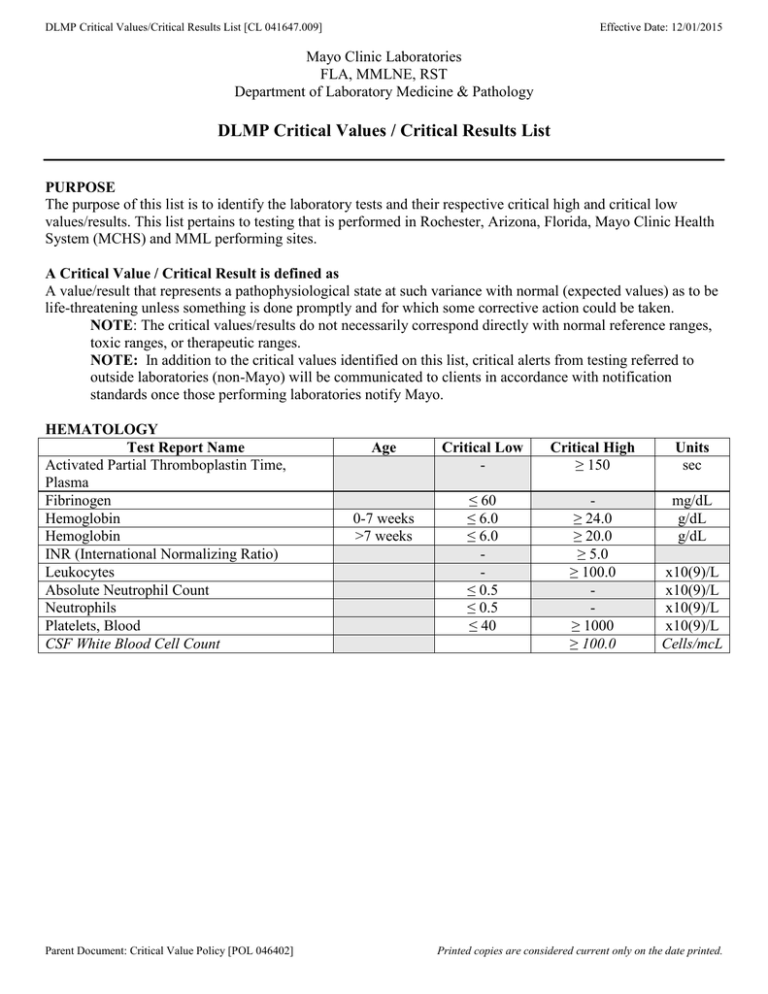
DLMP Critical Values/Critical Results List [CL 041647.009] Effective Date: 12/01/2015 Mayo Clinic Laboratories FLA, MMLNE, RST Department of Laboratory Medicine & Pathology DLMP Critical Values / Critical Results List PURPOSE The purpose of this list is to identify the laboratory tests and their respective critical high and critical low values/results. This list pertains to testing that is performed in Rochester, Arizona, Florida, Mayo Clinic Health System (MCHS) and MML performing sites. A Critical Value / Critical Result is defined as A value/result that represents a pathophysiological state at such variance with normal (expected values) as to be life-threatening unless something is done promptly and for which some corrective action could be taken. NOTE: The critical values/results do not necessarily correspond directly with normal reference ranges, toxic ranges, or therapeutic ranges. NOTE: In addition to the critical values identified on this list, critical alerts from testing referred to outside laboratories (non-Mayo) will be communicated to clients in accordance with notification standards once those performing laboratories notify Mayo. HEMATOLOGY Test Report Name Activated Partial Thromboplastin Time, Plasma Fibrinogen Hemoglobin Hemoglobin INR (International Normalizing Ratio) Leukocytes Absolute Neutrophil Count Neutrophils Platelets, Blood CSF White Blood Cell Count Parent Document: Critical Value Policy [POL 046402] Age 0-7 weeks >7 weeks Critical Low - Critical High ≥ 150 Units sec ≤ 60 ≤ 6.0 ≤ 6.0 ≤ 0.5 ≤ 0.5 ≤ 40 ≥ 24.0 ≥ 20.0 ≥ 5.0 ≥ 100.0 ≥ 1000 ≥ 100.0 mg/dL g/dL g/dL x10(9)/L x10(9)/L x10(9)/L x10(9)/L Cells/mcL Printed copies are considered current only on the date printed. DLMP Critical Values/Critical Results List [CL 041647.009] CHEMISTRY Test Report Name Ammonia – (Florida units are µmol/L, MCHS&RST units are mcmol/L) *Ammonia – Arizona (Deviation in units) Ammonia – (Florida units are µmol/L, Effective Date: 12/01/2015 Age ≥1 yr ≥1 yr < 1 yr Critical Low - Critical High ≥ 200 Units mcmol/L - ≥500 ≥ 100 mcg/dL mcmol/L ≤ 6.5 ≤ 2.0 ≤ 3.0 ≤ 3.0 ≥ 150 ≥ 15.0 ≥ 13.0 ≥ 6.0 ≥ 6.5 ≥ 5.5 mcg/dL mg/dL mg/dL mg/dL mg/dL mg/dL ≤ 3.0 ≥ 6.0 mg/dL - ≥ 20 % - ≥ 1.5 ≥ 2.0 mg/dL mg/dL - ≥ 2.5 ≥ 3.0 ≥ 10.0 ≥ 10,000 mg/dL mg/dL mg/dL U/L ng/dL MCHS&RST units are mcmol/L) *Ammonia – Arizona (Deviation in units) < 1 yr Bilirubin Total, Serum < 1 yr Calcium, Total Calcium, Ionized, Blood < 1 yr Calcium, Ionized, Blood ≥ 1 yr *Calcium, Ionized, Blood - Florida (Deviation < 1yr due to methodology difference) *Calcium, Ionized, Blood - Florida (Deviation ≥ 1 yr due to methodology difference) Carbon Monoxide (Carboxyhemoglobin Level) Creatinine, Blood/Plasma/Serum 1 day-4 weeks Creatinine, Blood/Plasma/Serum 5 weeks - 23 mos Creatinine, Blood/Plasma/Serum 2 yrs - 11 yrs Creatinine, Blood/Plasma/Serum 12 yrs -15 yrs Creatinine, Blood/Plasma/Serum ≥ 16 yrs Creatine Kinase, Total FT4 (Free Thyroxine) < 50 yrs FT4 (Free Thyroxine) FT4 (Free Thyroxine) – Florida Glucose, Plasma/Serum Glucose, Plasma/Serum Magnesium, Serum Osmolality *pH (MCHS and AZ only) *pC02, arterial (MCHS and AZ only) *pO2 (MCHS) *pO2 (AZ) Phosphorus Potassium Sodium Parent Document: Critical Value Policy [POL 046402] - - ≥ 7.8 ≥ 50 yrs All ages - ≥ < 4 weeks ≥ 4 weeks ≤ 40 ≤ 50 ≤ 1.0 ≤ 190 < 7.200 < 20.0 < 40.0 ≤ 45.0 ≤ 1.0 ≤ 2.5 ≤120 ≥ 7.8 6.0 ng/dL ng/dL > 400 ≥ 400 ≥ 9.0 ≥ 390 >7.600 >70.0 ≥ 6.0 ≥ 160 mg/dL mg/dL mg/dL mOsm/Kg pH mmHg mmHg mmHg mg/dL mmol/L mmol/L Printed copies are considered current only on the date printed. DLMP Critical Values/Critical Results List [CL 041647.009] TOXICOLOGY/TDM Test Report Name Acetaminophen, S Acetone (Volatile Screen), applies to all specimen types Amitriptyline and Nortriptyline, S Butalbital, S Caffeine, S Carbamazepine, Total, S Carbamazepine, Free, S Cyanide, B Desipramine, S Digoxin, S Disopyramide, S Doxepin and Nordoxepin, S Ethanol, Blood Ethanol, Serum Ethosuximide, S Ethylene Glycol, S Imipramine and Desipramine, S Isopropanol (Volatile Screen), applies to all specimen types Lidocaine, S Lead, Blood Lead, Blood Lithium, S Methanol (Volatile Screen), applies to all specimen types Nortriptyline, S Phenobarbital, S Phenytoin, Total, S Phenytoin, Free, S Primidone and Phenobarbital, S Primidone Phenobarbital Procainamide, S Procainamide N-Acetylprocainamide Quinidine, S Salicylates, S Theophylline, S Valproic Acid, Free and Total, S Free Valproic Acid Total Valproic Acid Valproic Acid, Total, S Parent Document: Critical Value Policy [POL 046402] Effective Date: 12/01/2015 Age Critical Low - Critical High > 150 4 hours after dose Units mcg/mL - Any value detected mg/dL - ≥ 300 ≥ 10 ≥ 30 ≥ 15.0 ≥ 4.0 ≥ 2.0 ≥ 300 ≥ 4.0 ≥ 7.0 ≥ 300 ≥ 400 ≥ 400 >150 ≥ 20 ≥ 300 ng/mL mcg/mL mcg/mL mcg/mL mcg/mL mcg/mL ng/mL ng/mL mcg/mL ng/mL mg/dL mg/dL mcg/mL mg/dL ng/mL mg/dL - Any value detected 0 – 15 yrs ≥ 16 yrs - > 6.0 ≥ 20 ≥ 70 > 1.6 Any value detected mcg/mL mcg/dL mcg/dL mmol/L mg/dL ≥ 300 ≥ 60.0 ≥ 30.0 ≥ 2.5 ng/mL mcg/mL mcg/mL mcg/mL ≥ 15.0 ≥ 60.0 mcg/mL mcg/mL >12 ≥ 40 ≥ 6.0 ≥ 50.0 >20 mcg/mL mcg/mL mcg/mL mg/dL mcg/mL >30 ≥ 151 ≥ 151 mcg/mL mcg/mL mcg/mL - - - Printed copies are considered current only on the date printed. DLMP Critical Values/Critical Results List [CL 041647.009] Effective Date: 12/01/2015 MICROBIOLOGY Result Detection (e.g., stain, culture, PCR, antigen detection) of a clinically significant bacterium, fungus, parasite, or virus (except HIV and hepatitis A through E virus) Identification/detection of a select agent (or other highly pathogenic organism) including, but not limited to Bacillus anthracis, Brucella species, Burkholderia mallei, Burkholderia pseudomallei, Clostridium botulinum, Corynebacterium diphtheriae, Coxiella burnetii, Francisella tularensis, monkeypox virus, variola virus, Vibrio cholerae, or Yersinia pestis. In the event of an outbreak of a novel contagious microorganism, detection of such an organism may fall into this category. Detection of clinically significant fungi including, but not limited to members of the Zygomycetes class, dimorphic fungal pathogens (Histoplasma capsulatum, Blastomyces dermatitidis, or Coccidioides species), Cryptococcus neoformans, Cryptococcus gattii, or Pneumocystis jiroveci Detection of Strongyloides stercoralis larvae Detection of herpes simplex virus or Bordetella pertussis Specimen source and patient details Blood, cerebrospinal fluid, brain tissue, amniotic fluid, ocular fluid/corneal scrapings Any specimen tested Any specimen tested Non-intestinal specimen Any specimen tested from a neonate (< 1 month) REVISION/DOCUMENT HISTORY Effective Date Version Synopsis of Change 10/25/2012 001 Created Mayo Enterprise Critical Value List and assigned document number 046404. This document replaces Mayo Clinic Rochester DLMP document number 027509 and replaces Arizona document DOCMAN-0000116209. 04/13/2015 002 Updated Ammonia and Valproic Acid. Approved by DLMP CPC 9/2014 08/24/2015 003 Updated Ammonia to reflect FLA unit of measure. Updated Free T4 (note: or ≥ upper limit of AMR if upper limit is <9.0 ng/dL) – approved by DLMP CPC 6/23/2015. Updated Acetaminophen, Ethosuximide, Procainamide, Theophylline, Free Valproic Acid - approved by DLMP CPC 3/23/2015. 12/01/2015 004 Changes to the Clinical Microbiology tests. Approved by CLMP CPC 4/9/2014, DLMP CPC 4/14/2014. Clarified note on Ammonia reporting units. Parent Document: Critical Value Policy [POL 046402] Printed copies are considered current only on the date printed.