Distributed Activation Energy Model of Heterogeneous Coal Ignition
advertisement

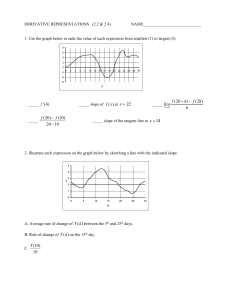
Distributed Activation Energy Model of Heterogeneous Coal Ignition John C. C. Chen Chen John We present a model that simulates the conventional used for conventional tube-furnace experiment .used f~r ignition i~ition studies. studi~s: The particle-to-partIcle variations vanatlOns in III reactivity reactivIty by Distributed Activation Activation Energy Model of Ignition accounts for particle-to-particle having having a single single preexponential factor and a Gaussian distribution of activation activation energies among am~ng the th~ particles. particles. III show that the model captures the key experimental experimental observations, observations, namely, namely, the linear increase Illcrease in The results show ignition frequency frequency with with increasing increasing gas temperature and the variation of the slope of the ignition ignition frequency frequen'!' with oxygen fit oxygen concentration. The article also also shows shows that adjustments to the model parameters permit a good fit experimental data. with experimental NOMENCLATURE N OMENCLATURE A0 d E E E0 Eo h He k n Q Q R S T y Y preexponential factor in in Arrhenius Arrhenius rate rate preexponential constant of of ignition ignition (kg m m--2Z s --l1)) constant diameter (m) diameter energy of of ignition ignition (kJ (kJ mol mol-I) activation energy t) mean of of Gaussian Gaussian distribution distribution mean (kJ mol-1 mol-- I )) (kJ heat transfer transfer coefficient convective heat m--2z K) ((W Wm heat of of reaction reaction (J kg --I) heat t) thermal conductivity conductivity (W (W m m--I thermal t K) order of ignition ignition with with respect respect reaction order reaction to oxygen heat generated or heat heat loss (W) (W) heat generated or universal gas constant constant (8.314 X × 1010 -33 kJ I 1 molmo1-1 KK -1)) external external surface area of particle particle (m Ze)) temperature temperature (K) molar ratio of CO CO to COz CO 2 Greek Greek Symbols Symbols X0 Xo22 6' E (T orbb orf i particle surface oxygen mole fraction at particle emissitivity of coal Stefan-Boltzmann S t e f a n - B o l t z m a n n constant constant (5.67 X × 4) 1010 _88 W mm -2Z KK -a) standard standard deviation of Gaussian distribution (kJ mol-I) mol- t) Subscripts Subscripts conv convection g gas p particle particle., rad radiation radiation IINTRODUCTION NTRODUCTION Numerous Numerous experiments experiments have have been been conducted conducted over over the the past past three three decades decades to to study study the the ignition of pulverized pulverized coals under under conditions conditions reletion of vant vant to utility boilers. boilers. The The conventional conventional experiment ment is based based on on one one developed developed by Cassel Cassel and and Liebman Liebman [1], and and consists of of a tube tube furnace furnace containing 0 2 and containing a heated heated mixture mixture of of Oz and inert inert gas. The e m p e r a t u r e of The ttemperature of the the furnace, and and hence hence the the gas, is the the independent independent variable in this arrangement. arrangement. The The experiment experiment is conducted conducted by dropping dropping a small batch batch of of presized presized coal partiparticles into into the the hot hot gas and and visually observing for ignition. In such a flash of light, which signals ignition. experiment the the particle particle concentration concentration is an experiment enough that that each particle can be typically low enough considered to behave independently independently of all othconsidered The furnace temperature temperature is then then deers. The creased and and the experiment experiment is repeated repeated to decreased the minimum m i n i m u m gas temperature (or the termine the which ignition occritical gas temperature) at which condition is termed termed critical ignition. curs. This condition particle size or the Oz 0 2 concentraconcentraFinally, the particle changed and, again, the critical ignition tion is changed condition is found for the new operating operating concondition dition. THEORY OF HETEROGENEOUS HETEROGENEOUS THEORY IGNITION IGNITION Essenhigh et al. [2] describe in detail the theEssenhigh heterogeneous ignition, that is, ignition ory of heterogeneous For a that occurs at the solid-gas interface. For coal particle exposed to an oxidizing environdetermined by the balance ment, ignition is determined between heat heat generation generation at at and and heat heat loss loss from between the particle particle surface. surface. The The heat heat loss loss from from the the the surface of of aa panicle particle at at temperature temperature Tp Tp is the the surface sum of of the the losses losses caused caused by convection and and sum radiation: radiation: surface surface is given by the the kinetic kinetic expression: expression: S (1) Equation 1 assumes assumes that that the the surroundings surroundings inEquation radiation exchange exchange are are in thermal thermal volved in radiation equilibrium with with the the gas. equilibrium The radiative radiative loss term term is relatively unimunimThe portant until until the the panicle particle temperature temperature exceeds portant 1500 K. K For For the the convective-loss term, we ~- 1500 assume that that the the Nusselt NusseIt number number equals equals 2, as is assume appropriate for very small particles, which leads leads appropriate = 2k /d 1 be • to hh = 2kg/dp. Thus, Eq. can be rewritten, g p on a per-external-surface-area basis, as: on Qlo~ S dQge, dQgen = dQlos dQloss~ dJ;, = ~ (6) (6) drp dJ;,' It is presumed presumed for the the purpose purpose of of this study that that certain of the the variables in Eqs. 2 and and 4 are are known a priori (He' (He, A0, A o, n, dp, d p , and and e), or or are are fixed by the experimental conditions (T (Tgg and and Xo2). Xo). The The values of these these variables used for the the base base case (described below) are are shown in Table Tpp and 1. The The remaining remaining unknowns, unknowns, T and E, can then then be determined determined by the the simultaneous simultaneous solution of of Eqs. 5 and and 6; the the relation of these two parameters to T Tgg for the base case is shown in parameters Tp manner repreFig. 1. T p determined in this manner sents the critical ignition temperature, whereas E can be interpreted as the critical (or maximum) activation energy that a particle may mum) under the given conditions. have and still ignite under A detailed expansion of Eqs. 5 and 6 is given in the Appendix. The gas thermal thermal conductivity, kg, in the The boundary layer around around a heated heated particle is boundary given by a linear fit to the conductivity of air over the temperature temperature range of 300-2000 300-2000 K: [T+T] (3) (3) Equation Equation 3 represents an approximation for the conductivity evaluated at the mean mean of the free-stream and particle-surface temperatures, temperatures, and it is noted that the variation of conductivity with temperature may be represented by higher order representations. The heat generated by a spherical carbon particle undergoing oxidation on its external M O D E L FORMULATION FORMULATION MODEL Figure 2 shows typical data [3] obtained from an ignition experiment conducted by varying the gas temperature while holding all other TABLE TABLEI Values Values of Parameters Parameters in in the Base Base Case Case of the Model Model Variable Variable Value Value A0 500 500 100 100 120 120 9210 9210 1.0 1.0 16.0 16.0 0.8 0.8 de E~ Hc n ~r e Units Units kgmkgm -22 s -1 S-I /Lm /~m kJ kJ mol-I mol- 1 kJ kJ kg-I kg- 1 -kJ kJ mol-I mol- 1 -- (4) (5) (5) Qgen =OJoss, 2kg (Tp - Tg) + eCro(Tp4 - Tg4). (2) dp W 5 Tp 7.0 X× 10lO_5[ W- • P + Tg] kkg== 7.0 g m K" g 2 mK . Diffusion effects are are neglected neglected because because at at the the relatively low particle of pulverparticle temperatures temperatures of ized-coal ignition, ignition, the the oxidation reaction reaction is kinetically controlled. At At the the critical ignition condition, the the following two conditions conditions are are satisfied [2]: Q10ss = = Oloss,conv Qloss,eonv + + QIoss, Qloss. rad rad Qloss = hS(T e - Tg) + ~o'bS(Tp 4 - rg'). = Hc x°2A°exp Remarks Remarks arbitrarily chosen chosen to to illustrate illustrate model model arbitrarily arbitrarily chosen chosen to illustrate illustrate model model arbitrarily arbitrarilychosen chosen to to illustrate illustrate model model arbitrarily for the the reaction reaction CC + 1/2 1/202 ~ CO CO for O 2 ..... arbitrarilychosen chosento to illustrate illustrate model model arbitrarily arbitrarilychosen chosen to to illustrate illustrate model model arbitrarily arbitrarilychosen chosento to illustrate illustrate model model arbitrarily 140 140 1300 1300 Q 1200 ~ 1200 ::l ~ 1100 1100 // ~~ 1000 1000 t-.I¢c: O 900 900 u / // / 120 /// E ~ / ~oo w 100 /~ / /// 70O 700 i'ii Ij :¥ 1/// 80 80 i 800 800 U 60 1200 1200 60O 600 600 6OO '7~ '0 / .E9 -~ 800 80o "~ :.e // // / :~c:e- "§ // // // // // // 1000 1000 T T~g (K) Fig. temperature (dashed Fig. 1. 1. Relation Relation of critical critical ignition ignition temperature (dashed line) temline) and critical critical activation activation energy energy (solid (solid line) line) to gas gas tembase case perature, TTg, for base case listed listed in Table Table 1. 1. g , for parameters constant. The parameters The data data shown was obthe experiment as detained by conducting the tained scribed earlier except that, at each temperarepeated ture, 10 to 20 tests for ignition were repeated to obtain obtain a frequency or probability of ignition. Fig. 2 shows that that ignition frequency increases approximately linearly with gas temperature, temperature, and and this is inconsistent with the heterogeneous ignition theory previously described. If If all particles of a coal sample sample used in an experiment have the same same reactivity, that that is, they are de100 100 , - - - . - - - - - - - N 80 80 ".e ~ "O -0 C1J 60 ct'~ ~ C1J "l ::J • ---, w .~ <Jl •• • • •• • • • • • 40 4o <Jl • ••• Cl.. 20 O • ---.___--_,_--__I o -+---~.... 700 750 800 850 900 Gas Gas Temperature T e m p e r a t u r e (K) (K) Fig. Fig. 2. 2. Typical Typical data from from a conventional conventional ignition ignition experiexperirelation between between ignition ment showing showing the relation ignition frequency frequency (or probability) and gas temperature for bituminous coal. probability) for a bituminous coal. Ref. 3. Data extracted extracted from from Ref. 3. scribed by a common Arrhenius Arrhenius rate constant as in Eq. 4, then then the data data would show an ignition frequency of 0% 0% until the critical gas temperature, corresponding to that temperature, that at the critical ignition condition, is reached. At any gas temperature above this, the ignition frequency temperature be 100%. Note that the observed igniwould be trend is not an artifact of the tion frequency trend experiment; this same behavior is reported from a variety of ignition experiments [4, 5, 6] including thermogravimetric analyzers and laser-based laser-based studies. One One of the reason why ignition frequency should increase gradually with increasing gas temperature is somewhat Within any temperature somewhat obvious: Within sample of coal, there exists a distribution of reactivity reactivity among the particles. Thus, in the conventional ignition experiment, in which a batch of perhaps perhaps a few hundred hundred particles of a batch sample is dropped dropped into the furnace, there is an increasing probability (or frequency) that at least one particle has a reactivity that that meets or exceeds the critical ignition condition set forth in Eqs. 5 and 6 as the gas temperature temperature is increased. Of Of course, there exist other other variations among among the particles within a sample, such as particle size and specific heat. Variation Variation in size alone could account for the observed increase in ignition frequency with gas temperature. It cannot account for another experimental observation, however, namely the variamental tion in the slope of the ignition frequency with oxygen concentration. (This behavior is described in a later section.) A distribution in specific heat heat would only affect the rate at which a particle attains its equilibrium temperature, temperature, but would not change this value or the reactivbut ity. Perhaps Perhaps other other variations could cause the observed behavior of ignition frequency. It is our premise premise that the distribution in reactivity dominates dominates all other other variations, however, and therefore accounts for the observed behavior. The Distributed Distributed Activation Energy Model of Ignition (DAEMI) (DAEMI) models the conventional ignition experiment by allowing for the particles within the coal sample sample to have a distribution of We prescribe that that all the particles reactivity. We the same properties, including the preexhave the ponential factor in the Arrhenius Arrhenius rate constant ponential constant describing their ignition reactivity, and and that their activation energy is distributed according to the Gaussian (or normal) distribution: 1 f ( E ) = (27rtr2)0. 5 exp - (E - 2tr2 RESULTS AND DISCUSSION E0) 2 (7) E 0 is the mean and IT 0" is the standard where Eo deviation of the distribution. The expression E+iJ>.E jE fee+ a E!(E) f ( E ) dE dE (8) describes the frequency or probability that particles within a sample have an activation en+ aE. A E. Accordingly, ergy in the range E to E + the distribution satisfies the condition that f~_~ !(E) f ( E ) dE dE = l. 1. r~-oo DAEMI divides a prescribed prescribed distribuThe DAEMI AE == 3 tion into discrete energy intervals of aE ld mol-I, tool-1, and considers only the energy range kJ E 0 --3 t3lT r to Eo E 0 ++3 3lT, o ' , rather than -00 - ~ to of Eo +o0. + 00. The latter simplification still covers 99.73% of the distribution. The model then calculates the frequency of being in each of these intervals by numerically integrating Eq. 8 for each of the intervals. An ignition experiment is modeled by assuming that 10 1055 particles are in the initial sample, and that they are distributed among the various AE aE intervals according to the calculated frequency for each interval. Each Each simulation of an experimental run under under a given set of conditions is conducted on a batch of 100 randomly selected particles from the sample, keeping in mind that no particle can be selected more more than once. Whether Whether or or not ignition occurs for a run is determined by the particle particle in the the batch batch of of 100 with the lowest activation energy. If If this particle's particle's reactivity equals equals or or exceeds that determined determined by the the critical ignition condition (that (that is, its activation energy is less than or equal equal to to the the critical than or energy determined determined by solution of of Eqs. 5 and and 6), the the batch batch is defined as ignited. This is consistent tent with our our observation observation [6] that that single-parsingle-particle ignition is discernible to the the eye, and and certainly to to a photon photon detector. This procedure procedure is repeated repeated 20 times at each each condition, just just as in actual actual experiments, experiments, to to determine determine an an ignition frequency at at this condition. Finally, the the gas temperature temperature is varied varied several several times and, and, each each time, 20 20 runs runs are are conducted. conducted. Figure 3 shows model results of ignition frequency versus gas temperature for one hypothetical sample for which Eo E 0 = 120 kJ mol-I, mo1-1, IT = 16 and A o0 ==5 500 or= 1 6 kkJ J mol-I, mo1-1,and 0 0 k gkgm m--22 S-I. s -1. The other parameters of this base case calculation are listed in Table 1. It is obvious that the DAEMI DAEMI exhibits the experimental characteristic of increasing ignition frequency with increasing gas temperature. As stated earlier, this is expected as an increase in gas temperature leads to an increase in the maximum activation energy that a particle can have and still be ignitable (see Fig. 1), and therefore to an increased probability of having at least one particle within each batch of a simulated run that is reactive enough to ignite. Figures 4 and 5 display the effects of varying E 0 and IT, or, respectively, on the DAEMI. DAEMI. An Eo increase in Eo, E0, which shifts the Gaussian distribution to higher energies (Fig. 4a), has the effect of shifting the ignition-frequency data to higher gas temperatures (Fig. 4b). This is the expected behavior as a representative batch from the higher Eo E 0 sample contains, on average, particles with higher activation energies, require a higher temperature temperature to induce which require Note that E Eo0 has only a slight effect ignition. Note on the slope of the data in Fig. 4b. 100 --.. >R.. ~ • •• 80 80 v •• >u ec 0) "1 :J 0" c- 660 0 • • O) u:,-. I.l_ c 440 0 0 O ¢c • • •• :-e o~ $ ._~ 20 0 • i 6600 00 A ~ w w i i 800 80O 11000 000 11200 200 T g (K) Tg Fig. 3. 3. RResults from D Distributed Activation Model Fig. e s u l t s from istributed A c t i v a t i o n EEnergy nergy M odel for bbase case ((Table 1). oof f IIgnition g n i t i o n ((DAEMI) D A E M I ) for a s e case T a b l e 1). 12000 , . . - - - - - - - - - - - - , 8000 <Jl a; 7000 (a) (a) <Jl a; 10000 10000 U 6000 .€ U °w '';:; 'n:l Q.. 8000 '- 4000 0 4000 a; 3000 t-~ .D E E 2000 '- 6000 '- '- Z Z Z Z n:l 0... 5000 0O a; .D E E ~ ~ I000 1000 2000 0 60 90 150 120 0+----..,..... 0 40 80 180 Activation Energy Activation Energy (kJ marl) mo1-1) 100 1oo ---- • • • 0~ ~ >u e-c: a; ~) -s ~ 0- a; 'u.. " cc: 0 O •~-e-- :-ec: 80 80 C 0 0 ~ (b) 160 • >- ••• 80 ~ u Q) • ~ ~ u.. " 0 20 20 • m 600 tC .-:: •~cc: , i 20 800 1000 0 1200 Tgg (K) T the effect Eo0 on Fig. 4. DAEMI D A E M I results showing s h o w i n g the effect of of E o n ignition frequency. tion frequency. (a) (a) Solid-colored S o l i d - c o l o r e d distribution d i s t r i b u t i o n corresponds corresponds to EEo0 = 110 kJ mo1-1, mol-I, and the distribution a n d the d i s t r i b u t i o n shown s h o w n in outline to EEo0 = 120 kJ m mol-I; pao u t l i n e corresponds c o r r e s p o n d s to o l - ~ ; all other o t h e r parrameters a m e t e r s are are as listed in Table T a b l e 1. (b) Ignition I g n i t i o n frequency frequency data Eo0 = mol- 1 ( Eo0 = mol-- l1 d a t a for for E = 110 kJ mo1-1 ( I.) ) and and E = 120 kJ tool (0). (o). Figure 5 shows that that an increase in the standard dard deviation (a ( o r)-the ) - - t h e spread spread of the GaussGaussian distribution (Fig. 5a)-has 5 a ) - - h a s two effects: a shift of the ignition frequency to lower temperatures and and a slower rise of ignition frequency temperature (Fig. 5b). These findings with gas temperature are somewhat because the average somewhat unexpected because both activation energy (Eo) ( E 0) is the same same for both the use of a samples, and and they result from the small batch batch (l00 (100 particles) in each run. The shift to lower temperatures temperatures is caused caused by the fact that, statistically, the most reactive particle in the larger atr batch will have a lower activa- " • " " • 00 0 O0 40 0O 0 •• • Q) '- 40 40 00 AA 60 6o 0- • (b) " " c: 0 200 --4~__, c- 60 60 0 100 ,----1oo ",.e 0 120 Activation Energy (kJ Activation (k] marl) tool -I) - 0 Jk 4000 " m i 600 800 800 A 1000 1000 1200 1200 Tgg (K) T the effect of Fig. 5. DAEMI D A E M I results showing s h o w i n g the of u o- on on ignition frequency. to frequency. (a) Solid-colored S o l i d - c o l o r e d distribution d i s t r i b u t i o n corresponds c o r r e s p o n d s to u mol--~,1, and the distribution cr = 20 kJ mol a n d the d i s t r i b u t i o n shown shown in outline outline mol corresponds to ut7 = 12 kJ m c o r r e s p o n d s to o l -~1I;; all other o t h e r pparameters a r a m e t e r s are are as listed in Table T a b l e 1. (b) Ignition I g n i t i o n frequency f r e q u e n c y data d a t a for u tr = = 20 u~ r == 12 mol--n1 (( A...)).. kkJJ mmolo l 1i ( ( I.) ) a nand d 1 2 kkJ J tool than the most reactive particle tion energy than batch because of its wider from the smaller aor batch spread spread in distribution. Thus, the higher reactivity allows for ignition to occur at lower temperThe wider spread spread and small batch atures. The batch also approach to 100% ignition cause the slower approach frequency because the probability of having a batch containing only relatively higher energy batch particles is increased, which requires higher gas temperatures temperatures until all runs result in igniparameters tion. Clearly, by adjusting adjusting the two parameters of the Gaussian Eo0 and Gaussian distribution distribution (Eq. 7), E and a, ~r, the DAEMI D A E M I can be fitted to ignition data, such that accurate valas those in Fig. 2, provided that ues for the parameters shown in Table 1 are available. D A E M I in its present form fails fails to The DAEMI capture a second experimental characteristic, namely, the change in slope of the ignition frequency data with oxygen concentration, as shown in Fig. 6. The lines shown are linear regressions of experimental data from Ref. 3. (The data points are explained below.) In fact, DAEMI shows a very weak dependence on the DAEMI oxygen concentration (not shown), so the quesparameter has not tion remains as to what parameter 100 100 50 50 25 -~" G ~ 0 v A- 0 IO0 100 Q) ~ ::J 25 25 Q) o0 100 100 :.ee'-c ~ ~ 1 I 1 kJ C+ + "20z z-&-O2 ~ ~ CO; H~,eo H~,co = 9,21O 9,210kg C kgC kJ C+ co 22 = 32,790+ Oz O2 ~ --* COz; CO2; H~ H~,co 32,790;gK , kg C C l -~ l y 1 Y H' 1 H'' He = Y y + + 1 H ' c,co + Y + 1 Hc, co2, c,eo + e,eo 2, l ~ 75 20o/002 50 50 25 0 100 f..•• 10% 75 0 2 50 50 25 25 00 i 600 600 700 700 • _.ff~O l i 8800 00 900 900 (9) Thus, the value of H H cc in Eq. 4 is dependent on the relative amounts of CO and COz C O 2 formed during ignition, and is given by the expression: 50010Oz 0 2 50"10 5o 50 0- u. u.. e'C .0 0 100% Oz 100"10 Oz .I °°" 75 o" ... eC ~ 4 75 been or is incorrectly accounted for. The obvious candidate is the reaction order, n, which must vary with X0 Xo22 in order to cause the observation. We can think of no fundamental basis for this behavior, however. A second, less obvious parameter parameter is the heat of reaction, H cc,' of ignition. So far in the presentation of the DAEMI, it has been assumed that the product of coal ignition is CO, as shown by the value for H H cc in Table 1. It is well known that the product of carbon oxidation is both CO and COz, CO 2, however, with the relative amounts dependent on both temperature and oxygen partial pressure [7, 8]. The significance of this is that their heats of reaction are vastly different: 1000 1000 TT8g (K) Fig. 6. Linear Linear regressions of of experimental experimental data data from Ref. Ref. 33 (shown as solid of free-stream solid lines), showing the the effect effect of oxygen concentration on ignition ignition of of aa high-volatile bitumibitumiconcentration on nous 5 - 9 0 /~m. h e data nous coal coal of of diameter diameter 775-90 p,m. T The data points points reprerepresent A E M I , including sent results results from from the the D DAEMI, including the the modification modification to O and to account account for for the the production production of of both both C CO and CO2 CO 2 and and adjustments 0, (r, u, and and n, n, as as adjustments to to the the base base case case values values for for EEo, described described in in the the text. text. (10) (10) where y = O / m o l COz' CO 2. We = mol C CO/mol We have asCO sumed here that energy released by any CO that oxidizes as it diffuses away from the particle surface does not affect the ignition. DAEMI should now This modification to the DAEMI Measurements show the experimental trend. Measurements that at higher particle particle temperature temperature [7, 8] show that Tg ), the the molar molar ratio (which results from higher Tg), CO/COz increases and and consequently He, H c ' the the C O / C O 2 increases of heat heat released released during ignition, deamount of amount creases. Therefore, Therefore, the the result of of a set of of runs creases. decreased oxygen level not only conducted at a decreased conducted the ignition frequency data data to a higher shifts the Tg (a (a direct result of of the decreased decreased Xo2) Xo) but but Tg reduces the slope of of the the rise (an (an indirect also reduces of the decreased decreased He). H c )' because of result because Direct measurements measurements of of the the C CO Direct O //CO C O 2z ratio Du et et al. [7] in a thermothermohave been been made made by Du have (TGA) using soot soot as the the gravimetric analyzer (TGA) carbon material. Measurements Measurements were were made made carbon of 667-873 667-873 K K and and over the the temperature temperature range range of over -, .g 0 >u >, c:: c (1) ::J "~ C" (1) L.. I,Ju.. c c:: 0o •~ c:: :-E tlO I O0 100 80 60 40 2o 20 0 100 100 80 80 60 40 40 20 0o 100 80 60 40 20 0 • 100% 02 • /. oZ e .6/_ 0 2 6 0 % 02 60% , 20% 02 0 2 • •• o ° ~ / • • •• 60O 600 800 800 I I 1000 1000 1200 1200 1400 1400 T Tg (K) (K) g Fig. 7. DAEMI results, including including the modification modification to acacFig. CO2, showing count for the production of both CO and CO 2 , showing effect of oxygen oxygen concentration on ignition ignition frequency. frequency. the effect The solid lines indicate linear regressions of the data points. oxygen partial partial pressures pressures from 0.1 to 1 atm. The The results at an oxygen partial partial pressure pressure of of 0.21 atm atm are are correlated by the expression: mo, CO [-3214] mol CO [ -3214] - - -2 = 59.95 59.95exp tool exp TpCK) Tp(K) J CO 2 mol CO (11) (11) This correlation is incorporated incorporated into into the the D A E M I and DAEMI and the the model model results results are are shown shown in Fig. 7. Notice Notice that that the the model model now now clearly possesses sesses the the desired desired characteristic. characteristic. Furthermore, Furthermore, it captures both the the decrease decrease in the the slope slope of of captures both the the ignition frequency with with decreasing decreasing oxygen concentration, of the the slope's slope's concentration, and and the the slow rate rate of decrease decrease until until low oxygen concentrations, concentrations, showing showing the the nonlinear nonlinear behavior behavior with Xo2 X0 2 displayed in experimental data (Fig. 6). experimental data By adjusting adjusting the the mean, mean, the the standard standard deviation tion and and the the reaction reaction order, order, n, n, of of the the base base case, A E M I can of D DAEMI can be be fit case, this this current current version version of to to the the experimental experimental data data shown shown in in Fig. Fig. 6. (The (The particle particle diameter diameter has has also also been been changed changed to to 83.0 JLm to match the mean of the sample used 83.0/xm in Ref. 3.) The model results using Eo E 0 == 84.0 1 1 , a kJ mol, and n = mo1-1, o- == 4.0 kJ molmol-1, = 0.4, are plotted as data points in Fig. 6 over the regression lines and show that a satisfactory fit fit is achieved with minimal effort in parameter adjustment, justment, despite the uncertainties in the values of other parameters. (Because of the small value of acr used, the energy intervals into which the distribution is divided was decreased from 3 kJ moltool 11 to 1 kJ molm o l - I1 to obtain these results.) It should be noted that the ignition parameters reported above represent merely a rough fit to the experimental data; it is certainly possible that another set of parameters can also fit the data satisfactorily, especially if a different value for A o0 were chosen. This extra effort may be worthwhile as certain parameters have strong theoretical justification (the reaction order, n, for example) for being within a particular range. We have begun work to examine this issue in more detail. Although Although we have assumed in this study that pulverized-coal ignition occurs heterogeheterogeneously without influence from any volatile matter that may be present, present, and even though the results closely fit the the experimental data, data, it the cannot be said that that the D DAEMI that cannot A E M I confirms that ignition is purely a heterogeneous process. Very models of homogeneous ignition have been been few models presented, and and none none have been been tested against against presented, the available experimental data data because because of the the inherent difficulty and and uncertainty in modeling modeling inherent devolatilization and and the combined solidsolid- and and gas-phase reactions. gas-phase CONCLUSIONS CONCLUSIONS The D DAEMI has been been formulated to model model The A E M I has It acconventional coal-ignition experiments. It counts for particle-to-particle variations variations in recounts within a sample sample by allowing for a disactivity within tribution in activation energies energies among among the the tribution particles and and a single preexponential preexponential factor. particles The model model captures captures the the main main characteristics characteristics The of actual actual experiments: the the gradual gradual increase increase in of increasing gas gas tempertemperignition frequency with increasing of the the slope slope of of the the ature and and the the variation variation of ature concentration. Fiignition frequency with with 0022 concentration. ignition has been been shown shown that that adjustments adjustments to to nally, it has the model model parameters parameters can can be be used used to to fit experithe mental data and extract reaction rate constants. of the U. U.S. of Energy The support of S. Department of DE-FG22-94MT94013) for this project is (Grant DE-FG22-94MT94013) gratefully acknowledged. Note that the neglect of the TTp dependence in p dependence kg k s introduces introduces a small error in Eq. 13. Following Eq. 6, we set Eq. 12 equal to Eq. E/RTp: 13 and solve for the quantity E/R~: 2k 4 2k gs y dpT zrp + + 4eubTp 4 e°'b Tr4 E E p = REFERENCES REFERENCES 1. Cassel, Cassel, H. M., M., and Liebman, Liebman, I.I. Combust. Combust. Flame Flame 1. 3:467-475 (1959). (1959). 3:467-475 2. Essenhigh, Essenhigh, R R. H., H., Mahendra, Mahendra, K K. M., M., and Shaw, Shaw, D. D. W., W., 2. Combust. Flame Flame 77:3-30 (1989). (1989). Combust. 3. Zhang, Zhang, D., D., Wall, Wall, T. T. F., F., Harris, Harris, D. D. J., J., Smith, Smith, I.I. W., W., 3. Chen, J., and Stanmore, Stanmore, B. B. R, R., Fuel Fuel 7:1239-1246 7:1239-1246 Chen, (1992). (1992). 4. Tomeczek, Tomeczek,J., and Wojcik, Wojcik, J., Twenty-Third Twenty-ThirdSymposium Symposium 4. (International) on on Combustion, Combustion, The Combustion Combustion InstiInsti(International) Pittsburgh, 1990, 1990, pp. 1163-1167. tute, Pittsburgh, Boukara, R, R., Gadiou, Gadiou, R, R., Gilot, Gilot, P., Delfosse, Delfosse, L., L., and 5. Boukara, Prado, G., G., Twenty-Fourth Twenty-FourthSymposium Symposium (International) (International)on Prado, Combustion, The Combustion Combustion Institute, Pittsburgh, Pittsburgh, Combustion, 1993, pp. 1127-1133. 1993, 6. Chen, Chen, J., Taniguchi, Taniguchi, M., M., Narato, K, K., and Ito, K, K., ComCorn6. bust. Flame Flame 97:107-117 97:107-117 (1994). (1994). bust. 7. Du, Z., Sarofim, Sarofim,A. E, F., and Longwell, Longwell, J. P., Energy Energyand 7. Fuels 5:214-221 5:214-221 (1991). (1991). Fuels 8. Mitchell, Mitchell, R R. E., Kee, Kee, R R. J., Glarborg, Glarborg, P., and Coltrin, Coltrin, 8. M. E., E., TwentyTwenty-Third Symposium (International) (International) on on M. Third Symposium Combustion, Combustion, The Combustion Combustion Institute, Pittsburgh, Pittsburgh, 1990, pp. 1169-1176. 1990, H c xd2Ao exp The The denominator denominator is recognized to be Qgen/S (Eq. 4), which by Eq. 5 is also QlossiS Qloss/S (Eq. 2). Thus Eq. 14 can be rewritten as: 2k 4 2ksg y drTp Tp + + 4eUb~ 4eCrbZp4 E E p RTr 2ks dr ( Tr - Tg) + eo'b(Tp4- Tg4) (15) (15) relation for E //RR~ T p is substituted substituted into the the This relation expression Q Q10ss = 0 to obtain a funcQgen Qtos~ = obtain gen tion, F, F, which is a function of T Tp p only: F(Tp) = = Received 1995; accepted Received 30 July July 1995; accepted 4 February February 1996 1996 (14) (14) [ Qgen -- Qloss Q10s s Qgen = He xg,~Ao Xo 2 A o = Hc APPENDIX APPENDIX Expansion Expansion of of Eqs. Eqs. 55 and and 66 In In order order to determine determine the the critical critical ignition ignition temperature and critical activaof the the particle, particle, Tr, and critical , perature of p tion E, Eqs. Eqs. 5 and and 6 are are solved simultasimultation energy, energy, E, neously. neously. Qgen Qgen and and Qtos~ Qloss are are given in in Eqs. Eqs. 22 and and 4, and and lead lead to to the the following derivatives with with respect respect to to temperature: temperature: dQgen dQsen E] (RT/ E) n c X~32Ao exp [ d~ = SH SHe Xo 2 A o exp RTp dQ 2k S dQtoss 2ks 3 ~ 4eUb STp. + 4eOrbSTp dTr = --gs dp + 3. dTp dp (12) (12) (13) (13) × exp 2ks - - C - r, - 4 br, . . . . -C(rr- r,) 4] ----~4- 4' r - r, ) (4 4) = O. 0. 2k(g T 2ks Tgg ) -- eO'b(Zp - subp T 4-- Zg Tg4) d ( T pr _- T) d rp (16) (16) The reasonable reasonable root root of of F(Tp) F(Tp ) corresponds corresponds to to The the critical critical ignition ignition temperature temperature of of the the partipartithe and substitution substitution of of this this value value into into Eq. Eq. 15 cle, and produces the the critical critical activation activation energy energy at at the the produces critical ignition ignition condition. condition. critical