Title: Hotter Boiling Water Objective: To demonstrate to students that
advertisement

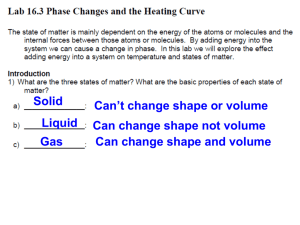
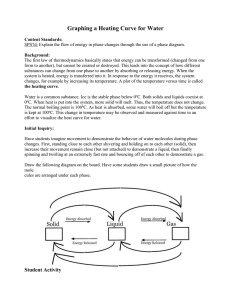
Title: Hotter Boiling Water Objective: To demonstrate to students that a beaker of boiling water initially has a temperature of 100° C, depending on elevation, but when salt or sugar is added to the water, the temperature rises well above 100° C. Purpose: To show that adding a water-soluble solid to water raises its boiling temperature. Standards: EALR 4 Physical Science: Big Idea: Force and Motion Materials: one 250 ml beaker, water, salt or sugar, one thermometer, one thermometer support, one spoon, one beaker support, one Bunsen burner, matches. Procedure: Place the beaker on the support and fill it with water. Carefully insert the thermometer in it and support the thermometer so that it doesn't touch the walls of the beaker. Light the burner and gently heat the beaker. Be careful not to heat the beaker too quickly or it may break. Soon the water will boil and the thermometer will read about 100° C. Now add a large spoonful of salt or sugar to the boiling water. Stir the mixture. When the mixture again begins to boil, the thermometer will read well above 100° C. Student Questions for Inquiry: 1. Explain what a water-soluble solid is. 2. Discuss what happens when the water-soluble solid is added to the water. Science Behind It: Adding a water soluble solid to water interferes with its ability to evaporate. With many of the water molecules involved in stabilizing the dissolved solid, there are fewer water molecules evaporating at any given temperature. The water temperature must exceed 100° C before evaporation is fast enough for evaporation bubbles to become stable within the body of the water so that boiling can occur.