C -- needs 4 e`s to complete its outer shell --
advertisement
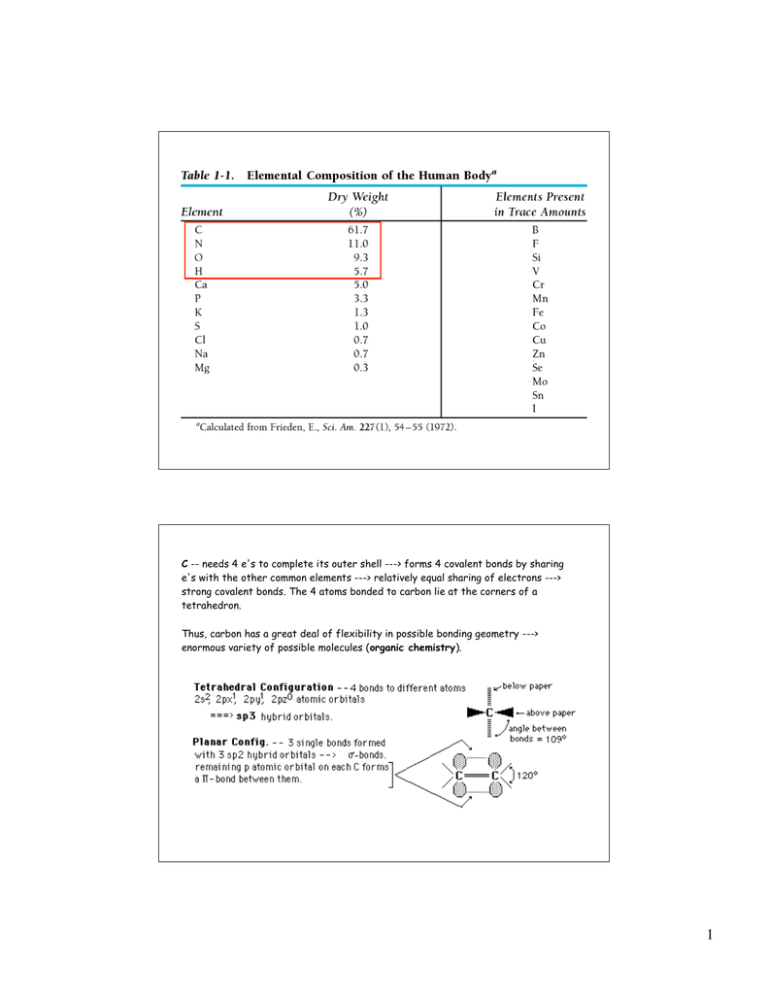
C -- needs 4 e's to complete its outer shell ---> forms 4 covalent bonds by sharing
e's with the other common elements ---> relatively equal sharing of electrons --->
strong covalent bonds. The 4 atoms bonded to carbon lie at the corners of a
tetrahedron.
Thus, carbon has a great deal of flexibility in possible bonding geometry --->
enormous variety of possible molecules (organic chemistry).
1
H -- needs 1 e' ---> one bond, e.g. H--H , --C--H etc.
O -- needs 2 e's ---> 2 bonds e.g. --C--O--H, H--O--H, >C==O
4 sp3 hybrid orbitals -- 2 have lone electron pairs and 2 form
covalent bonds
PRECURSOR MOLECULES: CH 4, CO 2, H 2O, NH 3, N 2, PO 4, SO 3, H 2S (MW's 18 -50) these were probably present in
the prebiotic earth and form...
BUILDING BLOCK MOLECULES: amino acids, nucleotides, monosaccharides (sugars), fatty acids, glycerol etc.
(MW's 100 - 400) these can be produced from precursors in simulations of prebiotic earth -- precursor molecules +
energy source such as electric discharge (lightening), radiation (X-ray, UV), heat
2
Princples of Thermodynamics
To analyze thermodynamic processes we divide the universe into two components
1.
2.
System: the part we're interested in
Surroundings: all of the rest
The System may be open and exchange matter and energy with the surroundings or it
may be close and cannot exchange matter and energy with the surroundings.
Living organisms are open systems.
First Law of Thermodynamics: Energy is Conserved
DU = Ufinal - Uinital = q - w
q = heat absorbed by the system from the surroundings
w = work done by the system on the surroundings
heat is random molecular motion while work is force times distanced moved under its influence
Exothermic Processes release heat and have q < 0
Endothermic Processes absorb heat and have q > 0
Energy: The SI unit is joule (J) although we will frequently use calorie ; 1 cal = 4.2 J
State Functions: are independent of the path or the way the system or universe gets to them. They
depend on the state of the system and not on its history. Energy, U, is a state function, therefore a cyclic
process (which returns to its original state) has DU = 0
Heat and work are not state functions because they depend on the path.
Enthalpy, H = U + PV is a useful function for living systems which operate at constant P & V (mostly).
DH =
qp the heat exchanged at constant P. Therefore at constant V DU
= DH.
Since qp is relatively easy to measure, DH for biological processes is relatively easy to measure
So what? Since DH is a State Function, we can measure it for any path and it will be valid for any other
path ==> the DH for glucose + O2 --> CO 2 + H2O can be measured in a calorimter and the value will be valid
for metabolism in a cell.
Second Law of Thermodynamics: The Universe tends toward Maximum Entropy
In any spontaneous process the disorder (Entropy) of the universe increases
(Universe = System + Surroundings). Disorder is defined as the number of equivalent ways, w, of
arranging the components. We define a measure of this called Entropy, S, which is more manageable
in expressing the many ways of arranging the components:
Entropy = S = k b ln W (kb = Boltzman's constant) and S is a State Function.
Since the energy of the universe is constant, the
For any spontaneous process --
2nd law of Thermodynamics is:
DSsystem + DSsurroundings = DSuniverse > 0
3
Free Energy: We can't measure DSuniverse easily and some processes occur when DSsystem < 0. We
need another indicator of spontaneity for the system.
at constant pressure, P, and temperature T: DS ≥ qP / T = DH / T
==> for a spontaneous process: DH - TDS < 0 first recognized by J. Willard Gibbs
so we call the Gibbs Free Energy, DG = DH - TDS and if DG < 0 a process will be spontaneous
A process which releases enough heat, DH < 0, can overcome a decrease in entropy of the system.
*
Processes with DG < 0 are exergonic and can be utilized to do work.
*
Processes with DG > 0 are endergonic and must be driven by the input of energy.
*
Processes with DG = 0 are at equilibrium. These are very important principles which we
will use frequently throughout this course.
DG < 0 does not mean that a process occurs rapidly. Proteins, Nucleic Acids, and
polysaccharides have DG's << 0 with respect to hydrolysis yet are perfectly stable in
the absence of enzymes (catalysts) to increase the speed of the process.
Chemical Equilibria: G depends upon concentration (S changes with concentration)
GA = GA° + RT ln[A]; GA° is the standard free energy of A, R = 2.0 cal/deg-mole = 8.3 J / deg-mole.
For the reaction aA + bB
cC = dD ==> DG
= DG o + RT ln
[C]c [D]d
= RT ln K eq
[A]a [B]b
DG° is the standard free energy change for this reaction when reactants and products
are in their standard states.
At equilibrium, DG = 0 ==>
†
DG o = -RT ln
[C]c [D]d
= RT lnK eq
[A]a [B]b
†
4
Standard States: By definition the free energy of pure elements in their standard
states (25°C, 1 atmosphere pressure, in their most stable form) is 0. The free energy
of formation of a compound, DGf, is the change in G for formation of one mole from
its component elements, all in their standard states.
Then for a chemical reaction DG° = DGf (products) - DGf (reactants).
Biochemists modify this definition to account for the fact that nearly all biochemical processes take
place in water near neutral pH, so the concentration of pure water, 55.5 M, and
[H+] = 1 x 10-7 M at pH = 7 are incorporated into DG° ==> DG°'
Coupled Reactions: An endergonic reaction can be driven by an exergonic
reaction; for example
A+B
C+D
DG1
D+E
F+G
DG2
A+B+E
F+G
DG3
DG3 = DG1 + DG2, so if |DG2| > |DG1| their sum, DG3 will be < 0 and the overall process
is spontaneous. Reaction 2 keeps [D] low so reaction 1 continues to make more. These
reactions are said to be couple by a common intermediate, D.
Biomolecules
What DETERMINES the PHYSICAL and CHEMICAL PROPERTIES of BIOMOLECULES??
A. Their Functional Groups:
Two functional groups can react together to form a new type of functional group; these reactions are very
important in the formation of biological molecules
a) esters -- an acidic group (carboxylic acid or organic phosphate) can react with an alcohol (or thiol) to form an ester:
b) amides -- an acidic group reacting with an amino group
B. Their Environment:
5
Fig. 2-1
d-
dd+
d+
dd+
dd+
Fig. 2-2: Hydrogen Bonds
6
Fig. 2-7: Polar Functional Groups
can form H-bonds with H2O
Fig. 2-6: The partial charges on H’s and O’s in H2O solvate dissolved ions
7
Fig. 2-5: van der Waal’s forces
are interactions between
permanent or induced dipoles.
8
HYDROPHOBIC EFFECT:
*
The tendency of water and hydrophobic molecules to minimize contact with each other
*
Drives the formation of 3D structures of proteins and membranes
This is spontaneous, therefore DG < 0; however in all cases DH > 0 so DS > 0 (i.e. -TDS < 0)
The Hydrophobic Effect or Hydrophobic Bond is Entropy Driven: Entropy decreases
when a hydrophobic molecule eneters water; i.e. the water becomes more ordered,
because H2O can't H-bond with hydrophobic molecules and forms an ordered cage
around them
Fig. 2-9
Fig. 2-10
9
Dissociation of H2O
H2O
H+ + OH-
[H + ][OH - ]
K=
[H 2O]
Since [H2O] remains essentially constant at 55 M
K[H 2O] = K w = [H + ][OH - ]
†
†
At 25°C Kw = 1 x 10-14 M2
In pure water at 25°C [H+] = [OH-] = 1 x 10-7 M
Thus, the concentrations of H+ and OH- we're concerned with in biological systems are
very small; but they can change by orders of magnitude (powers of 10). Therefore, we
want to express them on a LOGARITHMIC SCALE e.g.
pH = log10 {1 / [H+]} = - log10[H+] ==> at neutrality, pH = -log(10-7) = -(-7) = 7
the -sign makes physiological pH's positive
Bronsted -Lowry definitions: Acid --> Proton Donor and a Base --> Proton Acceptor
An example is Acetic Acid/Acetate anion: CH3COOH
CH3COO- + H+
Acetic acid is obviously a proton donor; Acetate is its conjugate base (proton acceptor)
because it can bind protons: CH3COO- + H2O ==> CH3COOH + OHFor Ammonia: NH3 + H2O
NH4+ + OH- and NH4+
NH3 + H+
Strong Acid: HCl ---> H+ + Cl- Strong Base: NaOH ---> Na+ + OHBoth completely dissociate producing either H+ (strong acids) or OH-(strong base) which
are the strongest acids and bases which can exist in aqueous solutions.
10
Weak Acid: HA
[HA]
H+
†
+
A-
===>
[H + ][A- ]
Ka =
[HA]
[A-]
Fig. 2-15: Titration curves
for acetic acid, phosphate,
and ammonia
11
Fig. 2-16: Titration of a polyprotic acid
12