Reflex Testing
advertisement
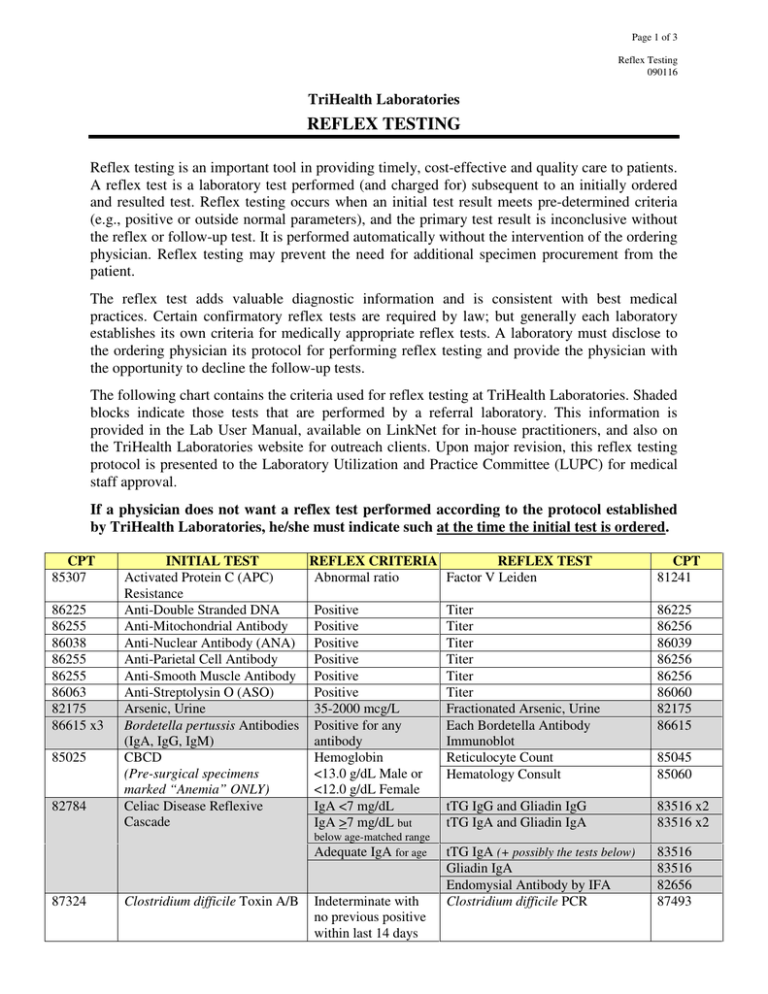
Page 1 of 3 Reflex Testing 090116 TriHealth Laboratories REFLEX TESTING Reflex testing is an important tool in providing timely, cost-effective and quality care to patients. A reflex test is a laboratory test performed (and charged for) subsequent to an initially ordered and resulted test. Reflex testing occurs when an initial test result meets pre-determined criteria (e.g., positive or outside normal parameters), and the primary test result is inconclusive without the reflex or follow-up test. It is performed automatically without the intervention of the ordering physician. Reflex testing may prevent the need for additional specimen procurement from the patient. The reflex test adds valuable diagnostic information and is consistent with best medical practices. Certain confirmatory reflex tests are required by law; but generally each laboratory establishes its own criteria for medically appropriate reflex tests. A laboratory must disclose to the ordering physician its protocol for performing reflex testing and provide the physician with the opportunity to decline the follow-up tests. The following chart contains the criteria used for reflex testing at TriHealth Laboratories. Shaded blocks indicate those tests that are performed by a referral laboratory. This information is provided in the Lab User Manual, available on LinkNet for in-house practitioners, and also on the TriHealth Laboratories website for outreach clients. Upon major revision, this reflex testing protocol is presented to the Laboratory Utilization and Practice Committee (LUPC) for medical staff approval. If a physician does not want a reflex test performed according to the protocol established by TriHealth Laboratories, he/she must indicate such at the time the initial test is ordered. CPT 85307 86225 86255 86038 86255 86255 86063 82175 86615 x3 85025 82784 INITIAL TEST REFLEX CRITERIA Activated Protein C (APC) Abnormal ratio Resistance Anti-Double Stranded DNA Positive Anti-Mitochondrial Antibody Positive Anti-Nuclear Antibody (ANA) Positive Anti-Parietal Cell Antibody Positive Anti-Smooth Muscle Antibody Positive Anti-Streptolysin O (ASO) Positive Arsenic, Urine 35-2000 mcg/L Bordetella pertussis Antibodies Positive for any (IgA, IgG, IgM) antibody CBCD Hemoglobin (Pre-surgical specimens <13.0 g/dL Male or marked “Anemia” ONLY) <12.0 g/dL Female Celiac Disease Reflexive IgA <7 mg/dL Cascade IgA >7 mg/dL but below age-matched range Adequate IgA for age 87324 Clostridium difficile Toxin A/B Indeterminate with no previous positive within last 14 days REFLEX TEST Factor V Leiden CPT 81241 Titer Titer Titer Titer Titer Titer Fractionated Arsenic, Urine Each Bordetella Antibody Immunoblot Reticulocyte Count Hematology Consult 86225 86256 86039 86256 86256 86060 82175 86615 85045 85060 tTG IgG and Gliadin IgG tTG IgA and Gliadin IgA 83516 x2 83516 x2 tTG IgA (+ possibly the tests below) Gliadin IgA Endomysial Antibody by IFA Clostridium difficile PCR 83516 83516 82656 87493 Page 2 of 3 Reflex Testing 090116 CPT 86900 86901 86880 INITIAL TEST Cord Blood Profile: • ABO Group • Rh Type • Direct Antiglobulin Test (DAT) 86403 89051 Cryptococcus Antigen CSF Cell Count (Emergency Dept. ONLY) Varies Culture REFLEX CRITERIA REFLEX TEST Rh Negative Du Antigen (Weak D) DAT Positive Type and Screen on maternal specimen Positive RBC >10/mcL and WBC <10/mcL on CSF Tube #3 or #4 Reflex testing depends on specimen and source CPT 86885 86900 86901 86850 and/or Antibody Elution on cord blood Titer Repeat Cell Count on CSF tube #1 86860 Antimicrobial Susceptibility 87186 or 87184 and/or Gram Stain and/or Anaerobic Culture Confirmation by GC/MS of each component as needed 87205 87075 86406 89050 80320 80324 80345 80347 80348 80349 80353 80354 80326 80345 80347 80349 80353 80356 80154 80184 82145 82520 82542 80356 80358 80359 80361 80365 80367 83992 80301 Drug Screen, Maternal Outpatient Positive 80301 Drug Screen, Serum or Plasma Positive Confirmation by GC/MS of each component as needed 80104 Drug Screen, Universal (Labor & Delivery ONLY) Positive Confirmation by GC/MS of each component as needed 83516 Endomysial Antibody, IgA Heavy Metals Panel 4 (or 6), Urine Positive tTG IgA Positive Arsenic Endomysial Antibody IgA Titer Fractionated Arsenic, Urine 86256 82175 Referral lab tests as determined by pathologist Sickle Cell Screen HBsAg Confirmation Confirmation by Molecular-SSP HIV 1 Antibody Confirmation by Western Blot HTLV I/II Confirmation by Western Blot Workup may include one or more: LA Hexagonal Phase LA Confirmation DVVT 50:50 Mix Borrelia burgdorferi Ab, IgG Confirmation by Western Blot Borrelia burgdorferi Ab, IgM Confirmation by Western Blot Varies 82175 83655 83825 82300 (+82525 +84630) 83020 85025 Hemoglobin Electrophoresis 87340 86812 87389 85730 85612 Hepatitis B Surface Antigen HLA B27 Human Immunodeficiency Virus (HIV) 1 & 2 Antibodies Human T-Lymphotropic Virus (HTLV) Types I/II Antibodies Lupus Anticoagulant (LA) • PTT • DVVT 86618 Lyme Antibodies, Total 86790 Unidentifiable abnormal band present S band present Reactive Indeterminate Reactive Positive Abnormal Positive 80358 80359 80361 80365 83992 83805 83840 83887 83925 83992 85660 87341 86812 86689 86689 85598 85613 85613 86617 86617 Page 3 of 3 Reflex Testing 090116 CPT 82664 84166 87430 86255 85461 86403 84432 86800 86780 84443 86900 86901 86850 81003 81003 INITIAL TEST REFLEX CRITERIA REFLEX TEST CPT Protein Electrophoresis, Serum Gamma Peak IgG, IgA, IgM 82784 x3 <0.7 g/dL Paraprotein present IgG, IgA, IgM, and Immunofix if 82784 x5 new patient not previously 86334 identified Protein Electrophoresis, Urine Paraprotein present Immunofix if new patient not 86335 previously identified Rapid Strep Group A Antigen Negative Strep Group A Nucleic Acid Probe 87650 Reticulin Antibody, IgA Positive Titer 86256 Rh Immunoglobulin Workup: • Fetal Cell Screen Positive Kleihauer-Betke Stain 85460 Strep Group A Antibody Positive Titer 86406 (Streptozyme) Thyroglobulin Thyroglobulin Thyroglobulin by LC-MS/MS 84432 antibody <4.0 IU/mL Treponema Antibody Positive or Equivocal RPR 86593 If RPR non-reactive, then T. pallidum Particle Agglutination 86780 84439 TSH with Reflex to Free T4 TSH > or < normal Free T4 range for patient’s age Type and Screen: Antibody Screen Workup may include any/all: • ABO Group Positive Antibody Identification Panel 86870 • Rh Type Direct Antiglobulin Test for 86880 x3 • Antibody Screen AHG, IgG, C3d Antigen Type patient RBCs 86905 (one antigen type per antibody identified) Antibody Elution 86860 Antibody Titer (pregnant patient, 86886 antibody is associated to HDFN) Hoxworth Reference Case 86999 If inpatient: • Antigen Type donor RBCs 86902 (one antigen type per antibody identified per unit) • Crossmatch pRBCs (per unit) 86922 Urinalysis Appearance not clear Urinalysis Replace (without microscopic exam) and/or positive (with microscopic exam) 81003 Protein, Blood, with Leukocyte Esterase 81001 or Nitrite Urinalysis Positive Leukocyte Urine Culture 87086 (Emergency Dept. ONLY) Esterase or Nitrite, or WBC >8/hpf REFERENCE HHS Office of Inspector General. Publication of OIG Compliance Program for Clinical Laboratories. Federal Register Notice, Vol. 63, No. 163, August 24, 1998, 45076-45087.


