Coulomb`s Law Packet
advertisement

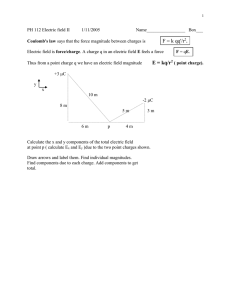
Physics Coulomb's Law Packet Name:___________ Coulomb’s Law of Electrostatics states that where: F is the force measured in Newtons k is Coulomb's constant which equals 9 x 109 Q is the magnitude of each charge in coulombs (remember electrons and protons are -/+ 1.6 x 10-19 C) r is the distance between the centers of the two charges in meters This formula may only be used for charges that are small and round. That is, for isolated points of electric charge. It is an example of an inverse square law. For example: if you double the distance between two charges then the force between them is reduced to ¼ of the original amount of force. If F is negative, that means that the charges carry opposite "signs" -- that is, one is positive and the other is negative. The negative answer means that the point charges are attracting each other -- it does NOT actually tell you in what direction the force is. If F is positive, that means that the charges carry the same "sign" -- that is, either both are positive or both are negative. A positive answer means that the point charges are repelling each other -- it does NOT actually tell you in what direction the force is. Coulomb’s Law is Essential in understanding the behavior of atoms which itself is vital in understanding chemistry and biology. Consider the hydrogen atom as a simple model below. 1. Which letter designates the electron orbiting the nucleus? AB 2. Which letter designates the proton in the nucleus? AB 3. Is the electron positively or negatively charged? _____________ 4. Is the proton positively or negatively charged? _____________ 5. The electrical interaction between the nucleus and the orbital electron is a force of ( attraction repulsion ). Using Coulombs Law answer these questions: 6. If the charge of either the nucleus or the orbital electron were greater, the force between the nucleus and the electron would be (greater less ). 7. If the distance between the nucleus and electron were greater the force would be ( greater less). 8. If the distance between the nucleus and electron were doubled, the force would be 1/4 as much 1/2 as much 2 times as much 4 times as much Refer to the following information for the next ten questions. Two charges, a +3x10-5C charge and a -6x10-5 C charge, are 2 meters apart. 9. What is the magnitude of the original electrostatic force between them? Show all work below. 10. Is it an attractive or repulsive force? ________ 11. How could you change the situation to reverse your answer to #10 ? _______________________________ 12. How would the original force change if the charges were moved twice as far apart? ___________________ Calculate the force below. 13. How would the original force change if the charges were moved towards each other to a final distance which equals half of their original separation? _______________ Calculate the force below. 14. How would the original force change if the charges were each doubled in size? _________________ Calculate the force below. 15. How would the original force change if each of the charges were to be cut in half? ________________ Calculate the force below. 16. How would the original force change if the charges were each doubled in size as well being moved to a distance that is twice their original separation? Explain. 17. Which of the following combinations of changes, involving both a change in the magnitude of the charges as well as their separation, could produce a force that is 64 times stronger than the original force between them? a) you could quadruple each charge and also move them to new positions which represent a distance that is only half of their original separation b) you could cut each charge in half and also move them to new positions which represent a distance that is 16 times greater than their original separation c) you could double one charge, quadruple the second charge and move them to new positions which represent a distance that is 8 times greater than their original separation d) you could double each charge and also move them to new positions which represents a distance that is only one-fourth their original separation justify your choice with a calculation 18. One Coulomb is actually an incredibly large amount of unbalanced charge. To see why imagine you have two Styrofoam balls left over from when you had to make a model of the solar system. If one coulomb of charge were placed on each ball, how far apart must the two balls be so that the force on these two balls is only 1 N (which is not very much force, it’s the weight of a small apple). Convert your answer to miles. 19. Two spheres A and B are charged so that A has twice the charge of B. The force between the two spheres is 0.5 N and they are 0.60 m apart. Find the charge of A and B. (Hint: call the charge of B “Q”) 20. A nucleus of uranium contains 92 protons. A) What is the charge in Coulombs of 92 protons? B) If two of these nuclei experience a force 0.22 N how far apart are they? Sample Problems: Given: Q1= 6.4 x 10-7 C Q2=7.3 x 10-7 C Felectric 0.3 N k 9 109 Nm2/C2 Unknown: r ? Equation: Solve for r: and then Substitute the values and solve: =0.12 m 2. Suppose you place equal but opposite amounts of charge at the Earth’s North and South Poles. How much charge is at each pole if the magnitude of the electric force compressing Earth is 5.17 105 N? Earth’s diameter is 1.27 107 m. Given: Felectric 5.17 105 N r 1.27 107 m k 9 109 Nm2/C2 Unknown: q ? Equation: Solve for Q since they are equal and then rearrange and finally Substitute the values into the equation(s) and solve: Even though the force is attractive (-) we don’t put the minus sign into the equation because we really don’t know what direction the force is.