05 Light Absorption in Semiconductors
advertisement
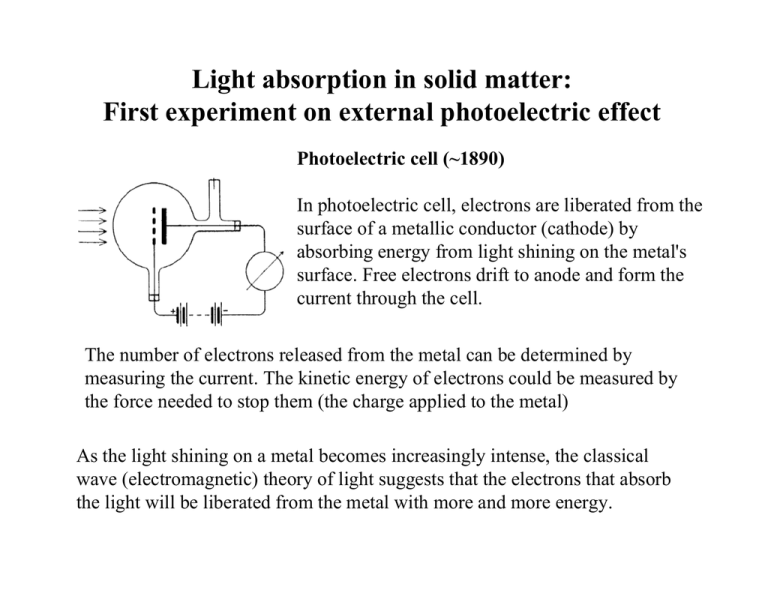
Light absorption in solid matter: First experiment on external photoelectric effect Photoelectric cell (~1890) In photoelectric cell, electrons are liberated from the surface of a metallic conductor (cathode) by absorbing energy from light shining on the metal's surface. Free electrons drift to anode and form the current through the cell. The number of electrons released from the metal can be determined by measuring the current. The kinetic energy of electrons could be measured by the force needed to stop them (the charge applied to the metal) As the light shining on a metal becomes increasingly intense, the classical wave (electromagnetic) theory of light suggests that the electrons that absorb the light will be liberated from the metal with more and more energy. In 1902, Phillip Lenard studied the photoelectric effect and discovered a number of key features: 1. The greater the intensity of the incoming light, the greater the number of electrons that were released. After all, when bigger, more powerful waves start hitting an ocean beach, they dislodge more sand than smaller, weaker waves. That observation made sense. 2. Next discovery did not make sense at all in the world of classical physics. He discovered that the kinetic energy contained in each of the escaping electrons did not increase when the intensity of the light increased. Where did the extra energy go? No answer. 3. Next discovery did not make sense at all in the world of classical physics. While increasing the intensity of light didn’t increase the energy of the released electrons, increasing the frequency of light (light color) did. Weaker waves with higher frequency supplied more energy to electrons than stronger waves with lower frequency. 4. Next discovery did not make sense at all in the world of classical physics. Light with low enough frequency could not release electrons from the metal no matter how strong it was. The photo-effect occurred only if the light frequency was above certain threshold value. Quantum nature of light Albert Einstein (1879-1955), probably the best-known scientist of the twentieth century, did not talk until he was three. However, even as a youth, he showed great mathematical ability. Einstein dropped out of school for a year when he was fifteen. He eventually attended the Swiss Federal Institute of Technology, but often cut classes; he passed by studying a classmate's notes. His professors refused to recommend him for an academic position— which is why he was working in the patent office in Bern, Switzerland, in 1905. Quantum nature of light In 1905, Einstein published a theory of the photoelectric effect. He believed that light and other forms of radiations are made up of discrete bundles of energy (particles, or quasi-particles), and that the energy of each bundle depends on the light’s frequency. This idea was soon supported by Max Planck’s theory, stating that the energy E of a single particle of light (photon) is given by: E = h ν, where h is a Plank constant and ν is the light frequency. In 1921, Albert Einstein received the Nobel Prize in Physics "for his services to Theoretical Physics, and especially for his discovery of the law of the photoelectric effect." Absorption Related electrical process: electron - hole pair generation The photon with the energy exceeding the bandgap energy of semiconductor can be absorbed. The photon disappears; the photon energy excites the electron from the valence band into the conduction band. As a result, one e-h pair is being created Photon absorption E = h ν = Εg Radiation Related electrical process: electron - hole pair recombination When the excited electron meets the hole in the valence band, it may occupy that place. As a result the e-h pair disappear; this process is called recombination. During recombination, the electron energy is released as a photon with the energy closed to the bandgap energy of the semiconductor. Photon Photon emission absorption E = h ν = Εg E = h ν = Εg Photon energy – wavelength relation E ph = h × ν = h × c λ Here h = 6.626 × 10-34 J×s; v = 3×108 m/s; Substituting the constants into the equation for Eph , we get: 1.24 Ε PH [eV ] = λ [ µm] Here, we have used: 1 eV = 1.6×10-19 J; 1 µm = 10-6 m Example: find the energy of the photon corresponding to the light wavelength of λ = 0.63 µm (RED LIGHT) Substituting the value of λ into the above expression for the photon energy, we find: Eph ≈ 1.97 eV; Photon energies, wavelengths and semiconductor bandgaps Eph ≈ 1.97 eV; What semiconductor material can absorb and emit the photon of such energy? Example problem The beam of red photons (λ=0.63 µm) creates an optical power density of 1 W/cm3 in a GaAs sample). The electron-hole life time is GaAs 5 µs. Find the concentration of photo generated electron-hole pairs Solution Optical power density = Photon energy × Number of photons per second per unit volume: Note: Number of photons per second per unit volume absorbed in the material, is the generation rate G Popt = Eph× G; G = Popt/Eph; Eph = 1.97 eV; G = (1W/cm-3) /(1.97*1.6e-19)J = 3.17 x 1018 cm-3 s-1. G = R = ∆n/τ; > ∆n = G x τ; ∆n = 3.17 x 1018 cm-3 s-1 x 5x10-6 s = 1.58x1013 cm-3 The energy and momentum conservation laws require certain conditions to be satisfied to make this absorption possible. Electron -- Photon Hole For the direct photon into e-h pair transformation, Eph = Eg; pph = | pe - ph|, where Eph = h× v is the photon energy, p is the momentum of the photon, of the electron or of the hole. The energy and momentum conservation laws require certain conditions to be satisfied to make this absorption possible. Eph = Eg; pph = | pe - ph|, The mass of the photon is negligibly small: pph ≈ 0; (because p = m×v) Therefore after the absorption the e and the h must have equal momentums: pe ≈ ph; The momentum of the quantum-mechanical particle of the mass m and velocity v is given by p = m×v = = k where k= 2π λ is the wave vector of the particle. Band diagram of direct-band semiconductor material and absorption/emission processes in such material: 1 – absorption 2 - emission Comments. A simple way to understand the semiconductor band diagram in energy – k coordinates is as follows. The kinetic energy of a free particle using a classic mechanics approach is 2 mv E= 2 Since m×v = = k , we expect that in quantum mechanical approximation, =2 k 2 E= 2m i.e. the E(k) dependence in the simplest case of free or quasi-free particle must be parabolic. This indeed is the case for some semiconductors (as shown above for the materials like GaAs, InP, GaN etc.) For other materials, for instance, Si, the dependence is more complicated: For the photon emission (top) or absorption (bottom), the THIRD particle IS NEEDED to meet the momentum conservation. This third particle is usually a PHONON (the crystal lattice vibration). Donor - Acceptor and Impurity-band Absorption Band – donor (a) and acceptor - band (b) absorption Low-energy (a) donor-band and (b) acceptor-band absorption Radiation in semiconductors In general, the reverse of all the absorption processes considered above can occur to produce radiation. 1) Band - to -band recombination (radiation) 2) Donor- acceptor recombination 3) Impurity band recombination. Not ANY recombination produces the RADIATION In case of indirect bands, the recombination produces phonons rather than photons. This is particularly the case of the most important semiconductor material, Si. Log-scale Absorption spectra