Physical quantities and units
advertisement
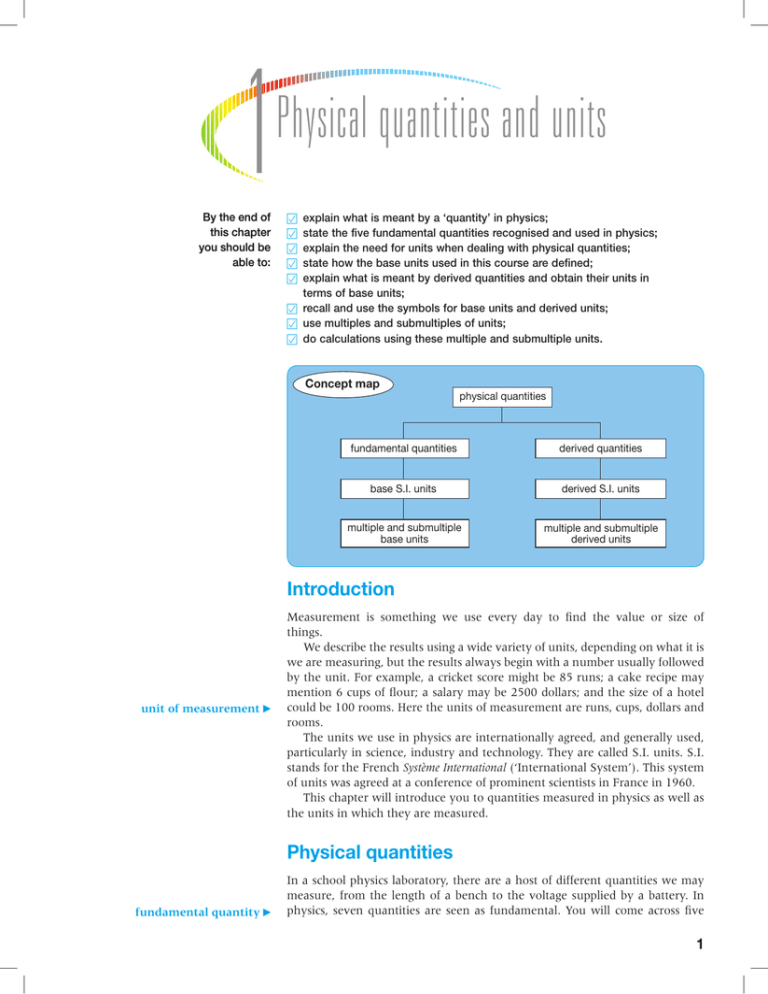
1 By the end of this chapter you should be able to: Physical quantities and units 嘼 嘼 嘼 嘼 嘼 explain what is meant by a ‘quantity’ in physics; state the five fundamental quantities recognised and used in physics; explain the need for units when dealing with physical quantities; state how the base units used in this course are defined; explain what is meant by derived quantities and obtain their units in terms of base units; 嘼 recall and use the symbols for base units and derived units; 嘼 use multiples and submultiples of units; 嘼 do calculations using these multiple and submultiple units. Concept map physical quantities fundamental quantities derived quantities base S.I. units derived S.I. units multiple and submultiple base units multiple and submultiple derived units Introduction unit of measurement Measurement is something we use every day to find the value or size of things. We describe the results using a wide variety of units, depending on what it is we are measuring, but the results always begin with a number usually followed by the unit. For example, a cricket score might be 85 runs; a cake recipe may mention 6 cups of flour; a salary may be 2500 dollars; and the size of a hotel could be 100 rooms. Here the units of measurement are runs, cups, dollars and rooms. The units we use in physics are internationally agreed, and generally used, particularly in science, industry and technology. They are called S.I. units. S.I. stands for the French Système International (‘International System’). This system of units was agreed at a conference of prominent scientists in France in 1960. This chapter will introduce you to quantities measured in physics as well as the units in which they are measured. Physical quantities fundamental quantity In a school physics laboratory, there are a host of different quantities we may measure, from the length of a bench to the voltage supplied by a battery. In physics, seven quantities are seen as fundamental. You will come across five 1 A – Measurement and practical work of the fundamental quantities in your course: mass (figure 1.1), length, time, temperature and electric current. (The other two fundamental quantities are ‘luminous intensity’ and ‘amount of substance’.) Each of these fundamental quantities is represented by a symbol, as shown in table 1.1. Table 1.1 Figure 1.1 Mass is a fundamental quantity in physics. The kilogram standard mass, shown here, is kept at Sèvres. base unit Fundamental quantity Symbol for the quantity mass m length l time t temperature T electric current I Units for fundamental quantities When we measure a quantity, we express the value as a number followed by a unit such as ‘metre’ or ‘second’. Each of the fundamental quantities in physics has an S.I. base unit. For example, the base unit of length is the metre. The base units are defined using internationally agreed standards. The five base units most often used in physics are shown in table 1.2 with their symbols. Table 1.2 In print, the symbols for quantities in physics are shown as here, in italic, for example T, not T. Symbols for units are never written in the plural. For example, we would write 10 kg, not 10 kgs. Five fundamental quantities and their symbols. Five fundamental quantities and their S.I. base units. Fundamental quantity Symbol for the quantity Base unit Symbol for the unit mass m kilogram kg length l metre m time t second s temperature T kelvin K electric current I ampere A The units kelvin and ampere are named after famous scientists. The kilogram The standards kept at the International Bureau of Weights and Measures are ‘primary’ standards. Other ‘standards’, made in properly equipped laboratories and based on those at the International Bureau, are called ‘secondary standards’. kilogram standard 2 The kilogram is the base unit of mass. The kilogram is defined as the mass of a particular platinum–iridium cylinder kept at the International Bureau of Weights and Measures at Sèvres, near Paris, in France, stored under specified conditions (figure 1.1). This cylinder is called the kilogram standard. All other masses are ultimately measured against this standard (figure 1.2). Figure 1.2 A high-precision balance. Values for mass are ultimately based upon the primary standard kilogram at Sèvres. 1 – Physical quantities and units Thus if we say that a certain mass is 40 kilograms, what we mean is that the mass is 40 times that of the kilogram ‘standard’. The mass of a standard must not change with time or with environmental conditions. The kilogram standard is made from an alloy chosen for its resistance to corrosion and is kept under very closely controlled conditions (figure 1.1). The metre and the second Figure 1.3 A caesium clock. Caesium clocks are so constant that two of them will agree with each other to within 1 second in 300 000 years! This means that if the two clocks were switched on at the same time, then after they had been working for a period of 300 000 years, the times they showed would differ by no more than 1 second! Measurements using a caesium clock show that the Earth’s daily rotation is not constant, but is very gradually slowing down. The values of base units must remain constant, irrespective of the environment. Because of this, the older definition of the metre, based on the separation of two fine scratches on a bar of a particular alloy, has had to be abandoned. In 1983, the metre was redefined as the distance travelled by light in a vacuum in 1/299 792 458 of a second. (You do not need to remember this number!) The older definition of the second, based on the rotation of the Earth on its axis, has also been abandoned. The second was redefined in 1967, as the time for 9 192 631 770 vibrations of a particular electromagnetic wave given off by the atoms of caesium-133 (figure 1.3). (You do not need to remember that number either!) When standardised in this way, the values of the metre and the second are not affected by environmental conditions. Further, these definitions allow the standards to be reproduced in any properly equipped laboratory anywhere in the world with the same accuracy. The kelvin and the ampere The standard definitions of the kelvin and the ampere are outside the scope of our course, but are given here for completeness. You do not have to remember them. Zero kelvin (0 K) is the absolute zero of temperature. The kelvin (K) is defined as 1/273.16 of the temperature at which water can coexist as liquid, solid and gas. The ice point 0 °C = 273.15 K on the Kelvin scale. The ampere is defined as the current which, if flowing in two straight and infinitely long parallel wires 1 metre apart in vacuum, would produce a force between them of 2 × 10–7 newtons per metre. Multiple and submultiple units ‘Sub’ means ‘lower than’, ‘less than’, ‘below’ or ‘under’. submultiple unit multiple unit Imagine that two students are asked to measure the thickness of a leaf of their exercise books. One student gives the thickness as 0.2 millimetre while the other expresses the result as 0.0002 metre. Which of these two statements gives one a better idea of the thickness? You may have a perfectly good idea of the size of a millimetre and of a metre, but it is more difficult to visualise two ten-thousandths of a metre than twotenths of a millimetre. So there is a need for units smaller than the base unit, and these are called submultiple units. We also need units of length that are greater than the metre. Imagine you are to run a marathon race. Is it easier to visualise a distance of 26 kilometres rather than 26 000 metres? Units such as the kilometre, which are larger than the fundamental unit, are called multiple units. There is a need for both multiple and submultiple units of most quantities. These units are the base unit multiplied by a power of 10. The factor by which the base unit is multiplied is given by a prefix, as shown in table 1.3. 3 A – Measurement and practical work Table 1.3 Prefixes for multiple and submultiple S.I. units. Prefix Submultiples The abbreviation da is hardly ever used. Hectoand deca- are also seldom used nowadays in physics. Multiples A hectare is a unit of area used for land measurement. One hectare is 10 000 m2, roughly 2.5 acres. micron Abbreviation Power of 10 pico p 10–12 nano n 10–9 micro µ 10–6 milli m 10–3 centi c 10–2 deci d 10–1 deca (or deka) da 101 hecto h 102 kilo k 103 mega M 106 giga G 109 tera T 1012 The unit ‘micrometre’ is sometimes called the micron, written as ‘µ’ (the Greek mu), without the m for ‘metre’. Liquid volumes in chemistry are commonly measured in dm3. 1 decimetre3 = 1 dm3 = (10–1 m)3 = 10–3 m3 This is 1 litre (l). The litre is used in chemistry and in commerce (figure 1.4). ITQ1 How many cm3 are there in 1 dm3? ITQ2 Express the following numbers in standard form: (i) 2000 (ii) 0.002 34 (iii) 3833.33 (iv) 0.000 000 02 (v) 123 456.789 ITQ3 Express (i) 10 000 milliseconds in seconds (ii) 2000 km in metres (iii) 2000 km in megametres (iv) 0.002 g in micrograms Take care to write the symbols correctly. 4 Figure 1.4 A filling station in Trinidad. What is the unit used on the pump for measuring the quantity of gasoline bought? Standard form This is a convenient way of writing very large or very small numbers, by expressing them as a number between 1 and 9.9999 that is multiplied by a power of 10. Standard form is also referred to as scientific notation. Examples are 6.02 × 1023 2.000 × 103 2 × 10–3 1 – Physical quantities and units Derived quantities derived quantity density Fundamental quantities can be multiplied or divided. For example, length (as in distance travelled) may be divided by time to find a speed. The resulting quantity, speed in this example, is called a derived quantity. Another derived quantity is density, which is mass per unit volume. density = mass volume Some other examples of derived S.I. quantities are shown in table 1.4. Table 1.4 derived unit Full stops are not used within units: we would write 5 m s–1, not 5 m. s.–1. We also leave a space between the m and the s, so we write m s–1, not ms–1, because ‘ms’ means ‘millisecond’. Some derived S.I. quantities. Derived quantity Unit Symbol Derivation acceleration metre per second squared m s–2 area metre squared m2 density kilogram per metre cubed kg m–3 electric charge coulomb C 1C = 1As energy joule J 1J = 1Nm force newton N 1 N = 1 kg m s–2 momentum kilogram metre per second kg m s–1 potential difference volt V 1 V = 1 J C–1 power watt W 1 W = 1 J s–1 pressure pascal Pa 1 Pa = 1 N m–2 = 1 kg m–1 s–2 velocity metre per second m s–1 volume metre cubed m3 for unit The units used to measure derived quantities are called derived units. For example, derived quantity, speed = distance travelled (length) time taken (time) derived unit of speed metre m = second s = So the unit of speed is the derived unit, m/s, or m s–1. We say that the unit of speed has the dimensions ‘metre/second’, or m s–1. Any unit obtained by multiplying or dividing base units is a derived unit. Units named after famous scientists newton Units are often named after scientists who have made a significant contribution to a particular field of study. For example, Isaac Newton did a lot of work in the area of mechanics, which is mostly about the effect of forces, and so the unit of force, the newton, has been named after him. The symbol for this unit is N. 5